Abstract
Main conclusion
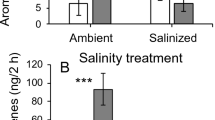
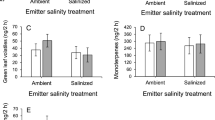
Salinity alters VOC profile in emitter sweet basil plants. Airborne signals by emitter plants promote earlier flowering of receivers and increase their reproductive success under salinity.
Abstract
Airborne signals can prime neighboring plants against pathogen and/or herbivore attacks, whilst little is known about the possibility that volatile organic compounds (VOCs) emitted by stressed plants alert neighboring plants against abiotic stressors. Salt stress (50 mM NaCl) was imposed on Ocimum basilicum L. plants (emitters, namely NaCl), and a putative alerting-priming interaction was tested on neighboring basil plants (receivers, namely NaCl-S). Compared with the receivers, the NaCl plants exhibited reduced biomass, lower photosynthesis, and changes in the VOC profile, which are common early responses of plants to salinity. In contrast, NaCl-S plants had physiological parameters similar to those of nonsalted plants (C), but exhibited a different VOC fingerprint, which overlapped, for most compounds, with that of emitters. NaCl-S plants exposed later to NaCl treatment (namely NaCl-S + NaCl) exhibited changes in the VOC profile, earlier plant senescence, earlier flowering, and higher seed yield than C + NaCl plants. This experiment offers the evidence that (1) NaCl-triggered VOCs promote metabolic changes in NaCl-S plants, which, finally, increase reproductive success and (2) the differences in VOC profiles observed between emitters and receivers subjected to salinity raise the question whether the receivers are able to “propagate” the warning signal triggered by VOCs in neighboring companions.








Similar content being viewed by others
Abbreviations
- A N :
-
Net photosynthesis
- F0/Fm/Fv :
-
Minimal/maximal/variable chlorophyll fluorescence yield in dark-adapted leaves
- g s :
-
Stomatal conductance
- PCA:
-
Principal component analyses
- VOC:
-
Volatile organic compound
- WUE:
-
Water use efficiency
References
Adams RP (1995) Identification of essential oil components by gas cromatography and mass spectroscopy. Allured Publishing Corp, Illinois
Agati G, Cerovic ZG, Pinelli P, Tattini M (2011) Light-induced accumulation of ortho-dihydroxylated flavonoids as non-destructively monitored by chlorophyll fluorescence excitation techniques. Environ Exp Bot 73:3–9
Ameye M, Allmann S, Verwaeren J, Smagghe G, Haesaert G, Schuurink RC, Audenaert K (2017) Green leaf volatile production by plants: a meta-analysis. New Phytol 220:666–683
Araniti F, Lupini A, Sunseri F, Abenavoli MR (2017) Allelopathic potential of Dittrichia voscosa (L.) W. Greuter mediated by VOCs: a physiologycal and metabolomic approach. PLoS One 12:e0170161
Baldwin IT (2010) Plant volatiles. Curr Biol 20:392–397
Baldwin IT, Schultz JC (1983) Rapid changes in tree leaf chemistry induced by damage: evidence for communication between plants. Sci 221:277–279
Bibbiani S, Colzi I, Taiti C, Guidi Nissim W, Papini A, Mancuso S, Gonnelli C (2018) Smelling the metal: volatile organic compound emission under Zn excess in the mint Tetradenia riparia. Plant Sci 271:1–8
Bolarín MC, Santa-Cruz A, Cayuela E, Pérez-Alfocea F (1995) Short-term solute changes in leaves and roots of cultivated and wild tomato seedlings under salinity. J Plant Physiol 147:463–468
Bouché N, Fait A, Bouchez D, Moller SG, Fromm H (2003a) Mitochondrial succinic-semialdehyde dehydrogenase of the γ-aminobutyrate shunt is required to restrict levels of reactive oxygen intermediates in plants. Proc Nat Acad Sci USA 100:6843–6848
Bouché N, Lacombe B, Fromm H (2003b) GABA signaling: a conserved and ubiquitous mechanism. Trends Cell Biol 13:607–610
Breitkreuz KE, Shelp BJ, Fischer WN, Schwacke R, Rentsch D (1999) Identification and characterization of GABA, proline and quaternary ammonium compound transporters from Arabidopsis thaliana. FEBS Lett 450:280–284
Caparrotta S, Boni S, Taiti C, Palm E, Mancuso S, Pandolfi C (2018) Induction of priming by salt stress in neighboring plants. Environ Exp Bot 147:261–270
Catola S, Centritto M, Cascone P, Ranieri A, Loreto F, Calamai L, Balestrini R, Guerrieri E (2018) Effects of single or combined water deficit and aphid attack on tomato volatile organic compound (VOC) emission and plant–plant communication. Environ Exp Bot 153:54–62
Ceccanti C, Landi M, Benvenuti S, Pardossi A, Guidi L (2018) Mediterranean wild edible plants: weeds or “new functional crops”? Molecules 23:2299
Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103:551–560
Cofer TM, Seidl-Adams I, Tumlinson JH (2018) From acetoin to (Z)-3-hexen-1-ol: the diversity of volatile organic compounds that induce plant responses. J Agric Food Chem 66:11197–11208
Covarrubias AA, Cuevas-Velazquez CL, Romero-Pérez PS, Rendón-Luna DF, Chater CCC (2017) Structural disorder in plant proteins: where plasticity meets sessility. Cell Mol Life Sci 74:3119–3147
Dicke M, Baldwin IT (2010) The evolutionary context for herbivore-induced plant volatiles: beyond the ‘cry for help’. Trends Plant Sci 15:167–175
Dudareva N, Klempien A, Muhlemann JK, Kaplan I (2013) Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol 198:16–32
El-Bassiouny HM, Bekheta MA (2005) Effect of salt stress on relative water content, lipid peroxidation, polyamines amino acids and ethylene of two wheat cultivars. Int J Agric Biol 7:363–368
Erb M (2018) Volatiles as inducers and suppressors of plant defense and immunity-origins, specificity, perception and signaling. Curr Opin Plant Biol 44:117–121
Fincheira P, Quiroz A (2018) Microbial volatiles as plant growth inducers. Microbiol Res 208:63–75
Forieri I, Hildebrandt U, Rostas M (2016) Salinity stress effects on direct and indirect defence metabolites in maize. Environ Exp Bot 122:68–77
Fougere F, Le Rudulier D, Streeter JG (1991) Effects of salt stress on amino acid, organic acid and carbohydrate composition of roots, bacteroids, and cytosol of alfa alfa (Medicago sativa L.). Plant Physiol 96:1228–1236
Goulas Y, Cerovic ZG, Cartelat A, Moya I (2004) Dualex: a new instrument for field measurements of epidermal ultraviolet absorbance by chlorophyll fluorescence. Appl Opt 43:4488–4496
Guerrieri E (2016) Who’s listening to talking plants? In: Ginwood R, Blande J (eds) Deciphering chemical language of plant communication. Signaling and communication in plants series. Springer, Basel, pp 117–136
Hoeberichts FA, Van Doorn WG, Vorst O, Hall RD, Van Wordragen MF (2007) Sucrose prevents up-regulation of senescence-associated genes in carnation petals. J Exp Bot 58:2873–2885
Jalali F, Zafari D, Salari H (2017) Volatile organic compounds of some Trichoderma spp. increase growth and induce salt tolerance in Arabidopsis thaliana. Fungal Ecol 29:67–75
Jones JB (1998) Plant nutrition manual. CRC Press, Boca Raton
Kathiresan A, Tung P, Chinnappa CC, Reid DM (1997) Gamma-aminobutyric acid stimulates ethylene biosynthesis in sunflower. Plant Physiol 115:129–135
Kegge W, Pierik R (2010) Biogenic volatile organic compounds and plant competition. Trends Plant Sci 15:126–132
Kessler A, Halitschke R, Poveda K (2011) Herbivory-mediated pollinator limitation: negative impacts of induced volatiles on plant–pollinator interactions. Ecology 92:1769–1780
Kinnersley AM, Turano FJ (2000) Gamma aminobutyric acid (GABA) and plant responses to stress. Crit Rev Plant Sci 19:479–509
Lähdesmäki P (1968) The amount of γ-amino butyric acid and the activity of glutamic decarboxylase in aging leaves. Physiol Plant 21:1322–1327
Landi M, Remorini D, Pardossi A, Guidi L (2013) Sweet basil (Ocimum basilicum) with green or purple leaves: which differences occur in photosynthesis under boron toxicity? J Plant Nutr Soil Sci 176:942–951
Lee K, Seo PJ (2014) Airborne signals from salt-stressed Arabidopsis plants trigger salinity tolerance in neighboring plants. Plant Signal Behav 9:e28392
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: measurement and characterization by UV–Vis spectroscopy. Curr Prot Food Anal Chem 1:F4–8
Loreto F, Schnitzler JP (2010) Abiotic stresses and induced BVOCs. Trends Plant Sci 15:154–166
Loreto F, Dicke M, Schnitzler JP, Turlings TCJ (2014) Plant volatiles and the environment. Plant Cell Environ 37:1905–1908
Lusebrink I, Evenden ML, Blanchet FG, Cooke JEK, Erbilgin N (2011) Effect of water stress and fungal inoculation on monoterpene emission from an historical and a new pine host of the mountain pine beetle. J Chem Ecol 37:1013–1026
Masclaux C, Valadier MH, Brugière N, Morot-Gaudry JF, Hirel B (2000) Characterization of the sink/source transition in tobacco (Nicotiana tabacum L.) shoots in relation to nitrogen management and leaf senescence. Planta 211:510–518
Matsui K (2016) A portion of plant airborne communication is endorsed by uptake and metabolism of volatile organic compounds. Curr Opin Plant Biol 32:24–30
Papadakis IE, Tsiantas PI, Tsaniklidis G, Landi M, Psychoyou M, Fasseas C (2018) Changes in sugar metabolism associated to stem bark thickening partially assist young tissues of Eriobotrya japonica seedlings under boron stress. J Plant Physiol 231:337–345
Pardossi A, Romani M, Carmassi G, Guidi L, Landi M, Incrocci L, Maggini R, Puccinelli M, Vacca W, Ziliani M (2015) Boron accumulation and tolerance in sweet basil (Ocimum basilicum L.) with green or purple leaves. Plant Soil 395:375–389
Pareja M, Qvarfordt E, Webster B, Mayon P, Pickett J, Birkett M, Glinwood R (2012) Herbivory by a phloem-feeding insect inhibits floral volatile production. PLoS One 7:e31971
Pompeiano A, Landi M, Meloni G, Vita F, Guglielminetti L, Guidi L (2017) Allocation pattern, ion partitioning, and chlorophyll a fluorescence in Arundo donax L. in responses to salinity stress. Plant Biosyst 151:613–622
Qualley A, Dudareva N (2001) Plant volatiles. Wiley, Chichester
Rai V, Vajpayee P, Singh SN, Mehrotra S (2004) Effect of chromium accumulation on photosynthetic pigments, oxidative stress defense system, nitrate reduction, proline level and eugenol content of Ocimum tenuiflorum L. Plant Sci 167:1159–1169
Ruther J, Kleier S (2005) Plant–plant signaling: ethylene synergizes volatile emission in Zea mays induced by exposure to (Z)-3-hexen-1-ol. J Chem Ecol 31:2217–2222
Shabala S, Munns R (2012) Salinity stress: Physiological constraints and adaptive mechanisms. In: Shabala S (ed) Plant stress physiology. CAB International, Oxford, pp 59–93
Sharkey TD, Yeh S (2001) Isoprene emission from plants. Annu Rev Plant Physiol Plant Mol Biol 52:407–436
Shelp BJ, Bown AW, McLean MD (1999) Metabolism and functions of gamma-aminobutyric acid. Trends Plant Sci 4:446–452
Sims JT, Kline JS (1991) Chemical fractionation and plant uptake of heavy metals in soils amended with co-composted sewage sludge. J Environ Qual 20:387–395
Singh M, Kumar J, Singh S, Singh VP, Prasad SM (2015) Roles of osmoprotectants in improving salinity and drought tolerance in plants: a review. Rev Environ Sci Biotech 14:407–426
Tarchoune I, Baâtour O, Harrathi J, Cioni PL, Lachaâl M, Flamini G, Ouerghi Z (2013) Essential oil and volatile emissions of basil (Ocimum basilicum) leaves exposed to NaCl or Na2SO4 salinity. J Plant Nutr Soil Sci 176:748–755
Urano K, Maruyama K, Ogata Y et al (2009) Characterization of the ABA-regulated global responses to dehydration in Arabidopsis by metabolomics. Plant J 57:1065–1078
Xia J, Sinelnikov IV, Han B, Wishart DS (2015) MetaboAnalyst 3.0—making metabolomics more meaningful. Nucleic Acids Res 43:251–257
Yoneya K, Takabayashi J (2014) Plant–plant communication mediated by airborne signals: ecological and plant physiological perspectives. Plant Biotechnol 31:409–416
Yuan JS, Himanen SJ, Holopainen JK, Chen F, Stewart CN (2009) Smelling global climate change: mitigation of function for plant volatile organic compounds. Trends Ecol Evol 24:323–331
Acknowledgements
This study in part was supported by the Italian Ministry of Education, University and Research (MIUR), project SIR-2014 cod. RBSI14L9CE (MEDANAT).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Landi, M., Araniti, F., Flamini, G. et al. “Help is in the air”: volatiles from salt-stressed plants increase the reproductive success of receivers under salinity. Planta 251, 48 (2020). https://doi.org/10.1007/s00425-020-03344-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-020-03344-y