Abstract
Main conclusion
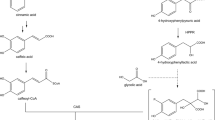
The nucleotide sequence of a BAHD hydroxycinnamoyltransferase was amplified from Actaea racemosa (Ranunculaceae) and expressed in E. coli. The protein catalysed the formation of cimicifugic acids and thus is named hydroxycinnamoyl-CoA:piscidic acid hydroxycinnamoyltransferase (ArHPT1; cimicifugic acid synthase).
Abstract
Actaea racemosa (syn. Cimicifuga racemosa) is known to contain triterpene lactone glycosides and cimicifugic acids. The latter are esters of various hydroxycinnamic or benzoic acids with piscidic or fukiic acid. Amplification of a nucleotide sequence from A. racemosa, that was already known as HCT1 from an EST approach, and its expression in E. coli resulted in a protein that was able to catalyse the formation of several cimicifugic acids. For the characterisation of this hydroxycinnamoyltransferase (hydroxy)cinnamoyl-coenzyme A thioesters were synthesised as donor substrates and piscidic acid isolated as acceptor substrate. The lowest Km-value with 6.8 µM was determined for p-coumaroyl-CoA. More than 30 possible acceptor substrates were tested, but only piscidic acid and putatively fukiic acid were accepted. The apparent Km-value for piscidic acid was 32.3 µM. High expression of the hydroxycinnamoyltransferase gene was found in roots, but the content of cimicifugic acids was higher in leaves and flowers than in roots. This work describes for the first time a biosynthetic step in the formation of cimicifugic acids catalysed by a so far uncharacterised hydroxycinnamoyltransferase accepting piscidic acid as acceptor substrate thus being a hydroxycinnamoyl-CoA:piscidic acid hydroxycinnamoyltransferase (ArHPT1; cimicifugic acid synthase).




Similar content being viewed by others
References
Berger A, Meinhard J, Petersen M (2006) Rosmarinic acid synthase is a new member of the superfamily of BAHD acyltransferases. Planta 224:1503–1510. https://doi.org/10.1007/s00425-006-0393-y
Beuerle T, Pichersky E (2002) Enzymatic synthesis and purification of aromatic coenzyme A esters. Anal Biochem 302:305–312. https://doi.org/10.1006/abio.2001.5574
Bontpart T, Cheynier V, Ageorges A, Terrier N (2015) BAHD or SCPL acyltransferase? What a dilemma for acylation in the world of plant phenolic compounds. New Phytol 208:695–707. https://doi.org/10.1111/nph.13498
Borrelli F, Izzo AA, Ernst E (2003) Pharmacological effects of Cimicifuga racemosa. Life Sci 73:1215–1229. https://doi.org/10.1016/s0024-3205(03)00378-3
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Chung IM, Lim JJ, Ahn MS, Jeong HN, An TJ, Kim SH (2016) Comparative phenolic compound profiles and antioxidative activity of the fruit, leaves, and roots of Korean ginseng (Panax ginseng Meyer) according to cultivation years. J Ginseng Res 40:68–75. https://doi.org/10.1016/j.jgr.2015.05.006
Cle C, Hill LM, Niggeweg R, Martin CR, Guisez Y, Prinsen E, Jansen MAK (2008) Modulation of chlorogenic acid biosynthesis in Solanum lycopersicum; consequences for phenolic accumulation and UV-tolerance. Phytochemistry 69:2149–2156. https://doi.org/10.1016/j.phytochem.2008.04.024
D’Auria JC (2006) Acyltransferases in plants: a good time to be BAHD. Curr Opin Plant Biol 9:331–340. https://doi.org/10.1016/j.pbi.2006.03.016
Dawson RF (1941) The localization of the nicotine synthetic mechanism in the tobacco plant. Science 94:396–397. https://doi.org/10.1126/science.94.2443.396
Dixon RA, Achnine L, Kota P, Liu CJ, Reddy MSS, Wang L (2002) The phenylpropanoid pathway and plant defence—a genomics perspective. Mol Plant Pathol 3:371–390. https://doi.org/10.1046/j.1364-3703.2002.00131.x
Elejalde-Palmett C, de Bernonville TD, Glevarec G, Pichon O, Papon N, Courdavault V, St-Pierre B, Giglioli-Guivarc’h N, Lanoue A, Besseau S (2015) Characterization of a spermidine hydroxycinnamoyltransferase in Malus domestica highlights the evolutionary conservation of trihydroxycinnamoyl spermidines in pollen coat of core Eudicotyledons. J Exp Bot 66:7271–7285. https://doi.org/10.1093/jxb/erv423
Erb M, Lenk C, Degenhardt J, Turlings TCJ (2009) The underestimated role of roots in defense against leaf attackers. Trends Plant Sci 14:653–659. https://doi.org/10.1016/j.tplants.2009.08.006
Gödecke T, Nikolic D, Lankin DC, Chen SN, Powell SL, Dietz B, Bolton JL, van Breemen RB, Farnsworth NR, Pauli GF (2009) Phytochemistry of cimicifugic acids and associated bases in Cimicifuga racemosa root extracts. Phytochem Anal 20:120–133. https://doi.org/10.1002/pca.1106
Hasa Y, Tazaki H (2004) Biosynthesis of fukinolic acid isolated from Petasites japonicus. Biosci Biotechnol Biochem 68:2212–2214. https://doi.org/10.1271/bbb.68.2212
He K, Pauli GF, Zheng B, Wang HK, Bai NS, Peng TS, Roller M, Zheng QY (2006) Cimicifuga species identification by high performance liquid chromatography-photodiode array/mass spectrometric/evaporative light scattering detection for quality control of black cohosh products. J Chromatogr A 1112:241–254. https://doi.org/10.1016/j.chroma.2006.01.004
Heller W, Tamm C (1975) Fukiinsäure und 3′-O-Methyl-fukiinsäure, zwei phenolische Hydroxycarbonsäuren aus Piscidia Erythrina. Helv Chim Acta 58:974–979. https://doi.org/10.1002/hlca.19750580403
Iwanaga A, Kusano G, Warashina T, Miyase T (2010a) Phenolic constituents of the aerial parts of Cimicifuga simplex and Cimicifuga japonica. J Nat Prod 73:609–612. https://doi.org/10.1021/np900752t
Iwanaga A, Kusano G, Warashina T, Miyase T (2010b) Hyaluronidase inhibitors from “Cimicifugae rhizoma” (a mixture of the rhizomes of Cimicifuga dahurica and C. heracleifolia). J Nat Prod 73:573–578. https://doi.org/10.1021/np900675n
Jennings AC (1981) The determination of dihydroxy phenolic-compounds in extracts of plant-tissues. Anal Biochem 118:396–398. https://doi.org/10.1016/0003-2697(81)90600-x
Kruse SO, Löhning A, Pauli GF, Winterhoff H, Nahrstedt A (1999) Fukiic and piscidic acid esters from the rhizome of Cimicifuga racemosa and the in vitro estrogenic activity of fukinolic acid. Planta Med 65:763–764. https://doi.org/10.1055/s-2006-960862
Kusano G, Takahira M, Shibano M, Kusano A, Okamoto Y, Tsujibo H, Numata A, Inamori Y (1998) Studies on inhibitory activities of fukiic acid esters on germination, α-amylase and carboxypeptidase A. Biol Pharm Bull 21:997–999. https://doi.org/10.1248/bpb.21.997
Laemmli UK (1970) Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227:680–685. https://doi.org/10.1038/227680a0
Mahmood T, Yang PC (2012) Western blot: technique, theory, and trouble shooting. N Am J Med Sci 4:429–434
Maier C, Conrad J, Carle R, Weiss J, Schweiggert RM (2015) Phenolic constituents in commercial aqueous Quillaja (Quillaja saponaria Molina) wood extracts. J Agric Food Chem 63:1756–1762. https://doi.org/10.1021/jf506277p
Nuntanakorn P, Jiang B, Einbond LS, Yang H, Kronenberg F, Weinstein IB, Kennelly EJ (2006) Polyphenolic constituents of Actaea racemosa. J Nat Prod 69:314–318. https://doi.org/10.1021/np0501031
Petersen MS (1991) Characterization of rosmarinic acid synthase from cell cultures of Coleus blumei. Phytochemistry 30:2877–2881. https://doi.org/10.1016/s0031-9422(00)98217-7
Petersen M (2016) Hydroxycinnamoyltransferases in plant metabolism. Phytochem Rev 15:699–727. https://doi.org/10.1007/s11101-015-9417-1
Petersen M, Simmonds MSJ (2003) Rosmarinic acid. Phytochemistry 62:121–125. https://doi.org/10.1016/s0031-9422(02)00513-7
Petersen M, Abdullah Y, Benner J, Eberle D, Gehlen K, Hücherig S, Janiak V, Kim KH, Sander M, Weitzel C, Wolters S (2009) Evolution of rosmarinic acid biosynthesis. Phytochemistry 70:1663–1679. https://doi.org/10.1016/j.phytochem.2009.05.010
Qiu F, McAlpine JB, Krause EC, Chen SN, Pauli GF (2014) Pharmacognosy of black cohosh: The phytochemical and biological profile of a major botanical dietary supplement. In: Kinghorn AD, Falk H, Kobayashi J (eds) Progress in the chemistry of organic natural products, vol 99. Springer, Cham, pp 1–68. https://doi.org/10.1007/978-3-319-04900-7_1
Rogers SO, Bendich AJ (1985) Extraction of DNA from milligram amounts of fresh, herbarium and mummified plant-tissues. Plant Mol Biol 5:69–76. https://doi.org/10.1007/bf00020088
Sakamura S, Yoshihara T, Toyoda K (1969) Fukiic acid isolated from a hydrolysate of a polyphenol in Petasites japonicus. Agric Biol Chem 33:1795–1797
Sakamura S, Yoshihara T, Toyoda K (1973) The constituents of Petasites japonicus: Structures of fukiic acid and fukinolic acid. Agric Biol Chem 37:1915–1921. https://doi.org/10.1271/bbb1961.37.1915
Sander M, Petersen M (2011) Distinct substrate specificities and unusual substrate flexibilities of two hydroxycinnamoyltransferases, rosmarinic acid synthase and hydroxycinnamoyl-CoA:shikimate hydroxycinnamoyltransferase, from Coleus blumei Benth. Planta 233:1157–1171. https://doi.org/10.1007/s00425-011-1367-2
Spiering MJ, Urban LA, Nuss DL, Gopalan V, Stoltzfus A, Eisenstein E (2011) Gene identification in black cohosh (Actaea racemosa L.): expressed sequence tag profiling and genetic screening yields candidate genes for production of bioactive secondary metabolites. Plant Cell Rep 30:613–629. https://doi.org/10.1007/s00299-010-0979-5
Stöckigt J, Zenk MH (1975) Chemical syntheses and properties of hydroxycinnamoyl-coenzyme A derivatives. Z Naturforsch 30c:352–358. https://doi.org/10.1515/znc-1975-5-609
St-Pierre B, De Luca V (2000) Evolution of acyltransferase genes: origin and diversification of the BAHD superfamily of acyltransferases involved in secondary metabolism. Rec Adv Phytochem 34:285–315
Sullivan ML (2009) A novel red clover hydroxycinnamoyl transferase has enzymatic activities consistent with a role in phaselic acid biosynthesis. Plant Physiol 150:1866–1879. https://doi.org/10.1104/pp.109.136689
Sullivan ML (2017) Identification of bean hydroxycinnamoyl-CoA:tetrahydroxyhexanedioate hydroxycinnamoyl transferase (HHHT): use of transgenic alfalfa to determine acceptor substrate specificity. Planta 245:397–408. https://doi.org/10.1007/s00425-016-2613-4
Tartoff KD, Hobbs CA (1987) Improved media for growing plasmid and cosmid clones. Bethesda Res Lab Focus 9:12
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets—procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354. https://doi.org/10.1073/pnas.76.9.4350
Tuominen LK, Johnson VE, Tsai CJ (2011) Differential phylogenetic expansions in BAHD acyltransferases across five angiosperm taxa and evidence of divergent expression among Populus paralogues. BMC Genom 12:236. https://doi.org/10.1186/1471-2164-12-236
Wang C, Zhang N, Wang Z, Qi Z, Zhu H, Zheng B, Li P, Liu J (2017) Nontargeted metabolomic analysis of four different parts of Platycodon grandiflorum grown in Northeast China. Molecules 72:1280. https://doi.org/10.3390/molecules22081280
Watanabe S, Hashimoto K, Tazaki H, Iwamoto Y, Shinohara N, Satoh K, Sakagami H (2007) Radical scavenging activity and inhibition of macrophage NO production by fukinolic acid, a main phenolic constituent in Japanese butterbur (Petasites japonicus). Food Sci Technol Res 13:366–371. https://doi.org/10.3136/fstr.13.366
Weitzel C, Petersen M (2011) Cloning and characterisation of rosmarinic acid synthase from Melissa officinalis L. Phytochemistry 72:572–578. https://doi.org/10.1016/j.phytochem.2011.01.039
Yannai S (2012) Dictionary of food compounds, 2nd edn. CRC, Boca Raton
Zenk MH (1979) Recent work on cinnamoyl-CoA derivatives. In: Swain T, Harborne JB, Van Sumere CF (eds) Recent advances in phytochemistry, vol 12. Biochemistry of plant phenolics. Plenum, New York, pp 139–176
Acknowledgements
We would like to thank Christiane Maier and Prof. Dr. Ralf Schweiggert (Universität Hohenheim) for sending us the extract of Quillaja saponaria, Prof. Dr. Guido Pauli (University of Illinois at Chicago) for a sample of fukinolic acid, Rixa Kraut (Universität Marburg) for performing LC–MS analyses and Elke Bauerbach and Olga Haag for technical assistance. The Bruker micrOTOF QIII mass spectrometer was financially supported in part by a grant from the Deutsche Forschungsgemeinschaft (INST 160/620-1) to Prof. Dr. Shu-Ming Li, Philipps-Universität Marburg.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Werner, V., Petersen, M. A BAHD hydroxycinnamoyltransferase from Actaea racemosa catalyses the formation of fukinolic and cimicifugic acids. Planta 250, 475–485 (2019). https://doi.org/10.1007/s00425-019-03181-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-019-03181-8