Abstract
Main conclusion
Glucomannan was more strongly oriented, in line with the orientation of cellulose, than the xylan in both compression wood and normal wood of Chinese fir. Lignin in compression wood was somewhat more oriented in the direction of the cellulose microfibrils than in normal wood.
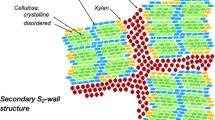
The structural organization in compression wood (CW) is quite different from that in normal wood (NW). To shed more light on the structural organization of the polymers in plant cell walls, Fourier Transform Infrared (FTIR) microscopy in transmission mode has been used to compare the S2-dominated mean orientation of wood polymers in CW with that in NW from Chinese fir (Cunninghamia lanceolata). Polarized FTIR measurements revealed that in both CW and NW samples, glucomannan and xylan showed a parallel orientation with respect to the cellulose microfibrils. In both wood samples, the glucomannan showed a much greater degree of orientation than the xylan, indicating that the glucomannan has established a stronger interaction with cellulose than xylan. For the lignin, the absorption peak also indicated an orientation along the direction of the cellulose microfibrils, but this orientation was more pronounced in CW than in NW, indicating that the lignin is affected by the orientation of the cellulose microfibrils more strongly in CW than it is in NW.





Similar content being viewed by others
Abbreviations
- CCD:
-
Charge-coupled device
- CW:
-
Compression wood
- FTIR:
-
Fourier transform infrared
- MFA:
-
Microfibril angle
- NW:
-
Normal wood
- RA:
-
Relative absorbance
- S1 :
-
Secondary cell wall layer, first layer
- S2 :
-
Secondary cell wall layer, middle layer
- S3 :
-
Secondary cell wall layer, third layer
- SEM:
-
Scanning electron microscope
- WAXS:
-
Wide-angle X-ray scattering
References
Åkerholm M, Salmén L (2001) Interactions between wood polymers studied by dynamic FT-IR spectroscopy. Polymer 42:963–969
Åkerholm M, Salmén L (2003) The oriented structure of lignin and its viscoelastic properties studied by static and dynamic FT-IR spectroscopy. Holzforschung 57:459–465
Altaner C, Hapca AI, Knox JP, Jarvis MC (2007a) Detection of β-1-4-galactan in compression wood of Sitka spruce [Picea sitchensis (Bong.) Carrière] by immunofluorescence. Holzforschung 61:311–316
Altaner C, Knox JP, Jarvis MC (2007b) In situ detection of cell wall polysaccharides in Sitka spruce (Picea sitchensis (Bong.) Carrière) wood tissue. BioResources 2:284–295
Altaner C, Tokareva EN, Jarvis MC, Harris PJ (2010) Distribution of (1-4)-β-galactans, arabinogalactan proteins, xylans and (1-3)-β-glucans in tracheid cell walls of softwoods. Tree Physiol 30:782–793
Andersson S, Wang Y, Pönni R, Hänninen T, Mononen M, Ren H, Serimaa R, Saranpää P (2015) Cellulose structure and lignin distribution in normal and compression wood of the Maidenhair tree (Ginkgo biloba L.). J Integr Plant Biol 57:388–395
Atalla RH, Agarwal UP (1985) Raman microprobe evidence for lignin orientation in the cell walls of native woody tissue. Science 227:636–638
Atalla RH, Hackney J, Uhlin I, Thompson N (1993) Hemicelluloses as structure regulators in the aggregation of native cellulose. Int J Biol Macromol 15:109–112
Bardage S, Donaldson L, Tokoh C, Daniel G (2004) Ultrastructure of the cell wall of unbeaten Norway spruce pulp fibre surfaces. Nordic Pulp Pap Res J 19:448–482
Boyd J (1982) An anatomical explanation for visco-elastic and mechano-sorptive creep in wood, and effects of loading rate on strength. In: Baas P (ed) New perspectives in wood anatomy. Martinus Nijhoff/DrWJunk Publishers, La Hague, pp 171–222
Brémaud I, Ruelle J, Thibaut A, Thibaut B (2013) Changes in viscoelastic vibrational properties between compression and normal wood: roles of microfibril angle and of lignin. Holzforschung 67:75–85
Burgert I, Frühmann K, Keckes J, Fratzl P, Stanzl-Tschegg S (2004) Structure-function relationships of four compression wood types: micromechanical properties at the tissue and fibre level. Trees 18:480–485
Busse-Wicher M, Gomes TC, Tryfona T, Nikolovski N, Stott K, Grantham NJ, Bolam DN, Skaf MS, Dupree P (2014) The pattern of xylan acetylation suggests xylan may interact with cellulose microfibrils as a twofold helical screw in the secondary plant cell wall of Arabidopsis thaliana. Plant J 79:492–506
Busse-Wicher M, Grantham NJ, Lyczakowski JJ, Nikolovski N, Dupree P (2016) Xylan decoration patterns and the plant secondary cell wall molecular architecture. Biochem Soc Trans 44:74–78
Cave ID (1997) Theory of X-ray measurement of microfibril angle in wood. Wood Sci Technol 31:143–152
Dammström S, Salmén L, Gatenholm P (2009) On the interactions between cellulose and xylan in biomimetic bacterial cellulose/glucuronoxylan nanocomposites. BioResources 4:3–14
Donaldson LA, Knox JP (2012) Localization of cell wall polysaccharides in normal and compression wood of radiata pine: relationships with lignification and microfibril orientation. Plant Physiol 158:642–653
Donaldson LA, Singh AP (2013) Structure and formation of compression wood. In: Fromm J (ed) Cellular aspects of wood formation plant cell monographs. Springer, Heidelberg, pp 225–256
Fackler K, Thygesen LG (2013) Microspectroscopy as applied to the study of wood molecular structure. Wood Sci Technol 47:203–222
Fagerstedt KV, Mellerowicz E, Gorshkova T, Ruel K, Joseleau J-P (2014) Cell wall polymers in reaction wood. The biology of reaction wood. Springer, Berlin, pp 37–106
Faix O (1991) Classification of lignins from different botanical origins by FT-IR spectroscopy. Holzforschung 45:21–28
Fengel D, Wegener G (1983) Wood: chemistry, ultrastructure, reactions. Walter de Gruyter, Berlin
Gindl W, Teischinger A (2003) Comparison of the TL-shear strength of normal and compression wood of european larch. Holzforschung 57:421–426
Grantham NJ, Wurman-Rodrich J, Terrett OM, Lyczakowski JJ, Stott K, Iuga D, Simmons TJ, Durand-Tardif M, Brown SP, Dupree R, Busse-Wicher M, Dupree P (2017) An even pattern of xylan substitution is critical for interaction with cellulose in plant cell walls. Nat Plants 3:859–865
Hänninen T, Tukiainen P, Svedström K, Serimaa R, Saranpää P, Kontturi E, Hughes M, Vuorinen T (2012) Ultrastructural evaluation of compression wood-like properties of common juniper (Juniperus communis L.). Holzforschung 66:389–395
Houtman CJ, Atalla RH (1995) Cellulose-lignin interactions (a computational study). Plant Physiol 107:977–984
Jungnikl K, Koch G, Burgert I (2008) A comprehensive analysis of the relation of cellulose microfibril orientation and lignin content in the S2 layer of different tissue types of spruce wood (Picea abies (L.) Karst.). Holzforschung 62:475–480
Kim JS, Awano T, Yoshinaga A, Takabe K (2010) Immunolocalization of β-1-4-galactan and its relationship with lignin distribution in developing compression wood of Cryptomeria japonica. Planta 232:109–119
Kim JS, Awano T, Yoshinaga A, Takabe K (2011) Occurrence of xylan and mannan polysaccharides and their spatial relationship with other cell wall components in differentiating compression wood tracheids of Cryptomeria japonica. Planta 233:721–735
Liang CY, Marchessault RH (1959) Infrared spectra of crystalline polysaccharides; II. Native celluloses in the region from 640 to 1700 cm−1. J Polym Sci 39:269–278
Liang CY, Basset KH, McGinnes EA, Marchessault RH (1960) Infrared spectra of crystalline polysaccharides; VII. Thin wood sections. Tappi 43:1017–1024
Marchessault RH (1962) Application of infra-red spectroscopy to cellulose and wood polysaccharides. Pure Appl Chem 5:107–130
Marchessault RH, Liang CY (1962) The infrared spectra of crystalline polysaccharides; VIII. Xylans. J Polym Sci 59:357–378
Meier H (1985) Localization of polysaccharides in wood cell walls. In: Higuchi T (ed) Biosynthesis and biodegradation of wood components. Academic press, Orlando, pp 43–50
Mottiar Y, Vanholme R, Boerjan W, Ralph J, Mansfield SD (2016) Designer lignins: harnessing the plasticity of lignification. Curr Opin Biotechnol 37:190–200
Olsson AM, Bjurhager I, Gerber L, Sundberg B, Salmen L (2011) Ultra-structural organisation of cell wall polymers in normal and tension wood of aspen revealed by polarisation FTIR microspectroscopy. Planta 233:1277–1286
Önnerud H (2003) Lignin structures in normal and compression wood. Evaluation by thioacidolysis using ethanethiol and methanethiol. Holzforschung 57:377–384
Paakkari T, Serimaa R (1984) A study of the structure of wood cells by X-ray diffraction. Wood Sci Technol 18:79–85
Placet V, Passard J, Perré P (2007) Viscoelastic properties of green wood across the grain measured by harmonic tests in the range 0–95 °C: hardwood vs. softwood and normal wood vs. reaction wood. Holzforschung 61:548–557
Salmén L (2015) Wood morphology and properties from molecular perspectives. Ann For Sci 72:679–684
Salmén L, Olsson A-M (1998) Interaction between hemicelluloses, lignin and cellulose: structure-property relationships. J Pulp Pap Sci 24:99–103
Salmén L, Olsson A-M, Stevanic JS, Simonović J, Radotić K (2012) Structural organisation of wood polymers in the wood fiber structure. BioResources 7:521–532
Savić A, Mitrović A, Donaldson L, Radosavljević JS, Pristov JB, Steinbach G, Garab G, Radotić K (2016) Fluorescence-detected linear dichroism of wood cell walls in juvenile Serbian spruce: estimation of compression wood severity. Microsc Microanal 22:361–367
Sedighi-Gilani M, Sunderland H, Navi P (2005) Microfibril angle non-uniformities within normal and compression wood tracheids. Wood Sci Technol 39:419–430
Sharma M, Altaner CM (2014) Properties of young Araucaria heterophylla (Norfolk Island pine) reaction and normal wood. Holzforschung 68:817–821
Simmons TJ, Mortimer JC, Bernardinelli OD, Pöppler AC, Brown SP, deAzevedo ER, Dupree R, Dupree P (2016) Folding of xylan onto cellulose fibrils in plant cell walls revealed by solid-state NMR. Nat Commun 7:13902
Simonović J, Stevanic J, Djikanović D, Salmén L, Radotic K (2011) Anisotropy of cell wall polymers in branches of hardwood and softwood: a polarized FTIR study. Cellulose 18:1433–1440
Sisson WA (1935) X-ray studies of crystalline orientation in cellulose fibers. Ind Eng Chem 27:51–56
Stevanic JS, Salmén L (2008) The primary cell wall studied by dynamic 2D FT-IR: Interaction among components in Norway spruce (Picea abies). Cell Chem Technol 40:761–767
Stevanic JS, Salmén L (2009) Orientation of the wood polymers in the cell wall of spruce wood fibres. Holzforschung 63:497–503
Terashima N (1990) A new mechanism for formation of a structurally ordered protolignin macromolecule in the cell wall of tree xylem. J Pulp Paper Sci 16:J150–J155
Terrett OM, Dupree P (2019) Covalent interactions between lignin and hemicelluloses in plant secondary cell walls. Curr Opin Biotechnol 56:97–104
Timell TE (1973a) Studies on opposite wood in conifers part II: histology and ultrastructure. Wood Sci Technol 7:79–91
Timell TE (1973b) Studies on opposite wood in conifers part I: chemical composition. Wood Sci Technol 7:1–5
Timell TE (1986) Compression wood in gymnosperms. Springer, Berlin
Acknowledgements
This research was sponsored by the National Key Research and Development Program of China (2017YFD0600202). Hui Peng has a fellowship from the China Scholarship Council (CSC). The authors wish to thank Jiali Jiang (Research Institute of Wood Industry of Chinese Academy of Forestry, China) and Liang Zhou (Anhui Agricultural University, China) for providing the wood samples.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Peng, H., Salmén, L., Stevanic, J.S. et al. Structural organization of the cell wall polymers in compression wood as revealed by FTIR microspectroscopy. Planta 250, 163–171 (2019). https://doi.org/10.1007/s00425-019-03158-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-019-03158-7