Abstract
Main conclusion
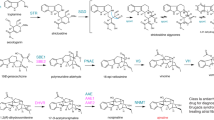
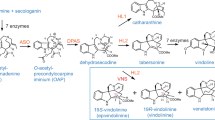
Based on findings described herein, we contend that the reduction of vomilenine en route to antiarrhythmic ajmaline in planta might proceed via an alternative, novel sequence of biosynthetic steps.
In the genus Rauvolfia, monoterpenoid indole alkaloids (MIAs) are formed via complex biosynthetic sequences. Despite the wealth of information about the biochemistry and molecular genetics underlying these processes, many reaction steps involving oxygenases and oxidoreductases are still elusive. Here, we describe molecular cloning and characterization of three cinnamyl alcohol dehydrogenase (CAD)-like reductases from Rauvolfia serpentina cell culture and R. tetraphylla roots. Functional analysis of the recombinant proteins, with a set of MIAs as potential substrates, led to identification of one of the enzymes as a CAD, putatively involved in lignin formation. The two remaining reductases comprise isoenzymes derived from orthologous genes of the investigated alternative Rauvolfia species. Their catalytic activity consists of specific conversion of vomilenine to 19,20-dihydrovomilenine, thus proving their exclusive involvement in MIA biosynthesis. The obtained data suggest the existence of a previously unknown bypass in the biosynthetic route to ajmaline further expanding structural diversity within the MIA family of specialized plant metabolites.






Similar content being viewed by others
Abbreviations
- MIAs:
-
Monoterpenoid indole alkaloids
- CAD:
-
Cinnamyl alcohol dehydrogenase
- VR:
-
Vomilenine reductase
- DHVR:
-
Dihydrovomilenine reductase
References
Almagro L, Fernández-Pérez F, Pedreño MA (2015) Indole alkaloids from Catharanthus roseus: bioproduction and their effect on human health. Molecules 20:2973–3000. doi:10.3390/molecules20022973
Asada K, Salim V, Masada-Atsumi S, Edmunds E, Nagatoshi M, Terasaka K, Mizukami H, De Luca V (2013) A 7-deoxyloganetic acid glucosyltransferase contributes a key step in secologanin biosynthesis in Madagascar periwinkle. Plant Cell 25:4123–4134. doi:10.1105/tpc.113.115154
Avrutina O, Schmoldt HU, Gabrijelcic-Geiger D, Le Nguyen D, Sommerhoff CP, Diederichsen U, Kolmar H (2005) Trypsin inhibition by macrocyclic and open-chain variants of the squash inhibitor MCoTI-II. Biol Chem 386:1301–1306. doi:10.1515/BC.2005.148
Bernhardt P, McCoy E, O’Connor SE (2007) Rapid identification of enzyme variants for reengineered alkaloid biosynthesis in periwinkle. Chem Biol 14:888–897. doi:10.1016/j.chembiol.2007.07.008
Brown S, Clastre M, Courdavault V, O’Connor SE (2015) De novo production of the plant-derived alkaloid strictosidine in yeast. Proc Natl Acad Sci USA 112:3205–3210. doi:10.1073/pnas.1423555112
Chao N, Liu SX, Liu BM, Li N, Jiang XN, Gai Y (2014) Molecular cloning and functional analysis of nine cinnamyl alcohol dehydrogenase family members in Populus tomentosa. Planta 240:1097–1112. doi:10.1007/s00425-014-2128-9
Cullen JK, Knees SG, Cubey HS (2011) The European garden flora. Angiospermae-Dicotyledons, vol IV. Cambridge University Press, Cambridge
Eurlings MC, Lens F, Pakusza C, Peelen T, Wieringa JJ, Gravendeel B (2013) Forensic identification of Indian snakeroot (Rauvolfia serpentina Benth. ex Kurz) using DNA barcoding. J Forensic Sci 58:822–830. doi:10.1111/1556-4029.12072
Fischbach MA, Clardy J (2007) One pathway, many products. Nat Chem Biol 3:353–355. doi:10.1038/nchembio0707-353
Gao S, von Schumann G, Stöckigt J (2002) A newly-detected reductase from Rauvolfia closes a gap in the biosynthesis of the antiarrhythmic alkaloid ajmaline. Planta Med 68:906–911. doi:10.1055/s-2002-34935
Geu-Flores F, Sherden NH, Courdavault V, Burlat V, Glenn WS, Wu C, Nims E, Cui Y, O’Connor SE (2012) An alternative route to cyclic terpenes by reductive cyclization in iridoid biosynthesis. Nature 492:138–142. doi:10.1038/nature11692
Góngora-Castillo E, Buell CR (2013) Bioinformatics challenges in de novo transcriptome assembly using short read sequences in the absence of a reference genome sequence. Nat Prod Rep 30:490–500. doi:10.1039/c3np20099j
Góngora-Castillo E, Childs KL, Fedewa G, Hamilton JP, Liscombe DK, Magallanes-Lundback M, Mandadi KK, Nims E, Runguphan W, Vaillancourt B, Varbanova-Herde M, DellaPenna D, McKnight TD, O’Connor SE, Buell CR (2012a) Development of transcriptomic resources for interrogating the biosynthesis of monoterpene indole alkaloids in medicinal plant species. PLoS One 7(12):e52506. doi:10.1371/journal.pone.0052506
Góngora-Castillo E, Fedewa G, Yeo Y, Chappell J, DellaPenna D, Buell CR (2012b) Chapter Seven—Genomic approaches for interrogating the biochemistry of medicinal plant species. Methods Enzymol 517:139–159. doi:10.1016/B978-0-12-404634-4.00007-3
Guo DM, Ran JH, Wang XQ (2010) Evolution of the cinnamyl/sinapyl alcohol dehydrogenase (CAD/SAD) gene family: the emergence of real lignin is associated with the origin of bona fide CAD. J Mol Evol 71:202–218. doi:10.1007/s00239-010-9378-3
Ibdah M, Berim A, Martens S, Valderrama AL, Palmieri L, Lewinsohn E, Gang DR (2014) Identification and cloning of an NADPH-dependent hydroxycinnamoyl-CoA double bond reductase involved in dihydrochalcone formation in Malus × domestica Borkh. Phytochemistry 107:24–31. doi:10.1016/j.phytochem.2014.07.027
Kim SJ, Kim MR, Bedgar DL, Moinuddin SG, Cardenas CL, Davin LB, Kang C, Lewis NG (2004) Functional reclassification of the putative cinnamyl alcohol dehydrogenase multigene family in Arabidopsis. Proc Natl Acad Sci USA 101:1455–1460. doi:10.1073/pnas.0307987100
Lewis NG, Yamamoto E (1990) Lignin: occurrence, biogenesis and biodegradation. Annu Rev Plant Physiol Plant Mol Biol 41:455–496. doi:10.1146/annurev.pp.41.060190.002323
Li L, Cheng XF, Leshkevich J, Umezawa T, Harding SA, Chiang VL (2001) The last step of syringyl monolignol biosynthesis in angiosperms is regulated by a novel gene encoding sinapyl alcohol dehydrogenase. Plant Cell 13:1567–1586
Linsmaier EM, Skoog F (1965) Organic growth factor requirements of tobacco tissue cultures. Physiol Plant 18:100–127
Miettinen K, Dong L, Navrot N, Schneider T, Burlat V, Pollier J, Woittiez L, van der Krol S, Lugan R, Ilc T, Verpoorte R, Oksman-Caldentey KM, Martinoia E, Bouwmeester H, Goossens A, Memelink J, Werck-Reichhart D (2014) The seco-iridoid pathway from Catharanthus roseus. Nat Commun 5:3606. doi:10.1038/ncomms4606
Panjikar S, Stöckigt J, O’Connor SE, Warzecha H (2012) The impact of structural biology on alkaloid biosynthesis research. Nat Prod Rep 29:1176–1200. doi:10.1039/C2np20057k
Rosenthal C, Mueller U, Panjikar S, Sun L, Ruppert M, Zhao Y, Stöckigt J (2006) Expression, purification, crystallization and preliminary X-ray analysis of perakine reductase, a new member of the aldo–keto reductase enzyme superfamily from higher plants. Acta Crystallogr Sect F Struct Biol Cryst Commun 62:1286–1289. doi:10.1107/S174430910605041X
Salim V, Yu F, Altarejos J, De Luca V (2013) Virus-induced gene silencing identifies Catharanthus roseus 7-deoxyloganic acid-7-hydroxylase, a step in iridoid and monoterpene indole alkaloid biosynthesis. Plant J 76:754–765. doi:10.1111/tpj.12330
Salim V, Wiens B, Masada-Atsumi S, Yu F, De Luca V (2014) 7-Deoxyloganetic acid synthase catalyzes a key 3 step oxidation to form 7-deoxyloganetic acid in Catharanthus roseus iridoid biosynthesis. Phytochemistry 101:23–31. doi:10.1016/j.phytochem.2014.02.009
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Sengupta D, Naik D, Reddy AR (2015) Plant aldo–keto reductases (AKRs) as multi-tasking soldiers involved in diverse plant metabolic processes and stress defense: a structure–function update. J Plant Physiol 179:40–55. doi:10.1016/j.jplph.2015.03.004
Staniek A, Bouwmeester H, Fraser PD, Kayser O, Martens S, Tissier A, van der Krol S, Wessjohann L, Warzecha H (2013) Natural products—modifying metabolite pathways in plants. Biotechnol J 8:1159–1171. doi:10.1002/biot.201300224
Staniek A, Bouwmeester H, Fraser PD, Kayser O, Martens S, Tissier A, van der Krol S, Wessjohann L, Warzecha H (2014) Natural products—learning chemistry from plants. Biotechnol J 9:326–336. doi:10.1002/biot.201300059
Stavrinides A, Tatsis EC, Foureau E, Caputi L, Kellner F, Courdavault V, O’Connor SE (2015) Unlocking the diversity of alkaloids in Catharanthus roseus: nuclear localization suggests metabolic channeling in secondary metabolism. Chem Biol 22:336–341. doi:10.1016/j.chembiol.2015.02.006
Stöckigt J, Zenk MH (1977) Strictosidine (isovincoside): the key intermediate in the biosynthesis of monoterpenoid indole alkaloids. J Chem Soc Chem Commun 18:646–648. doi:10.1039/C39770000646
Stöckigt J, Zenk MH (1995) Biosynthesis in Rauvolfia serpentina—modern aspects of an old medicinal plant. In: Cordell GA (ed) The alkaloids. Chemistry and pharmacology. Academic Press, San Diego, pp 115–172
Stöckigt J, Pfitzner A, Firl J (1981) Indole alkaloids from cell suspension cultures of Rauwolfia serpentina benth. Plant Cell Rep 1:36–39. doi:10.1007/BF00267656
Sun L, Ruppert M, Sheludko Y, Warzecha H, Zhao Y, Stöckigt J (2008) Purification, cloning, functional expression and characterization of perakine reductase: the first example from the AKR enzyme family, extending the alkaloidal network of the plant Rauvolfia. Plant Mol Biol 67:455–467. doi:10.1007/s11103-008-9331-7
von Schumann G, Gao S, Stöckigt J (2002) Vomilenine reductase—a novel enzyme catalyzing a crucial step in the biosynthesis of the therapeutically applied antiarrhythmic alkaloid ajmaline. Bioorg Med Chem 10:1913–1918
Warzecha H, Obitz P, Stöckigt J (1999) Purification, partial amino acid sequence and structure of the product of raucaffricine-O-β-d-glucosidase from plant cell cultures of Rauwolfia serpentina. Phytochemistry 50:1099–1109
Youn B, Kim SJ, Moinuddin SG, Lee C, Bedgar DL, Harper AR, Davin LB, Lewis NG, Kang C (2006) Mechanistic and structural studies of apoform, binary, and ternary complexes of the Arabidopsis alkenal double bond reductase At5g16970. J Biol Chem 281:40076–40088. doi:10.1074/jbc.M605900200
Zhu H, Kerčmar P, Wu F, Rajendran C, Sun L, Wang M, Stöckigt J (2015) Using strictosidine synthase to prepare novel alkaloids. Curr Med Chem 22:1880–1888
Acknowledgments
We are indebted to Markus Krischke for his help with LC–MS analysis. The support of COST Action FA1006 is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
M. Geissler and M. Burghard contributed equally to the work.
Rights and permissions
About this article
Cite this article
Geissler, M., Burghard, M., Volk, J. et al. A novel cinnamyl alcohol dehydrogenase (CAD)-like reductase contributes to the structural diversity of monoterpenoid indole alkaloids in Rauvolfia . Planta 243, 813–824 (2016). https://doi.org/10.1007/s00425-015-2446-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-015-2446-6