Abstract
Main conclusion
Strigolactone changes and cross talk with ABA unveil a picture of root-specific hormonal dynamics under stress.
Abstract
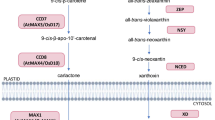
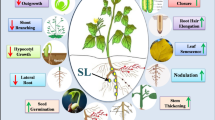
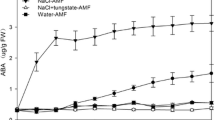
Strigolactones (SLs) are carotenoid-derived hormones influencing diverse aspects of development and communication with (micro)organisms, and proposed as mediators of environmental stimuli in resource allocation processes; to contribute to adaptive adjustments, therefore, their pathway must be responsive to environmental cues. To investigate the relationship between SLs and abiotic stress in Lotus japonicus, we compared wild-type and SL-depleted plants, and studied SL metabolism in roots stressed osmotically and/or phosphate starved. SL-depleted plants showed increased stomatal conductance, both under normal and stress conditions, and impaired resistance to drought associated with slower stomatal closure in response to abscisic acid (ABA). This confirms that SLs contribute to drought resistance in species other than Arabidopsis. However, we also observed that osmotic stress rapidly and strongly decreased SL concentration in tissues and exudates of wild-type Lotus roots, by acting on the transcription of biosynthetic and transporter-encoding genes and independently of phosphate abundance. Pre-treatment with exogenous SLs inhibited the osmotic stress-induced ABA increase in wild-type roots and down-regulated the transcription of the ABA biosynthetic gene LjNCED2. We propose that a transcriptionally regulated, early SL decrease under osmotic stress is needed (but not sufficient) to allow the physiological increase of ABA in roots. This work shows that SL metabolism and effects on ABA are seemingly opposite in roots and shoots under stress.







Similar content being viewed by others
Abbreviations
- ABA:
-
Abscisic acid
- AAO:
-
Abscisic-aldehyde oxidase
- ABC:
-
ATP-binding cassette
- AMF:
-
Arbuscular mycorrhizal fungi
- CCD:
-
Carotenoid cleavage dioxygenase
- D:
-
Dwarf
- DAD:
-
Decreased apical dominance
- EST:
-
Expressed sequence tag
- Ljccd7 :
-
CCD7-knocked-down L. japonicus
- MAX:
-
More axillary growth
- NCED:
-
Nine-cis-epoxycarotenoid dioxygenase
- PDR:
-
Pleiotropic drug resistance
- SL:
-
Strigolactone
References
Abe S, Sado A, Tanaka K, Kisugi T, Asami K, Ota S, Kim HI, Yoneyama K, Xie X, Ohnishi T, Seto Y, Yamaguchi S, Akiyama K, Yoneyama K, Nomura T (2014) Carlactone is converted to carlactonoic acid by MAX1 in Arabidopsis and its methyl ester can directly interact with AtD14 in vitro. Proc Natl Acad Sci USA 111:18084–18089
Akiyama K, Matsuzaki K, Hayashi H (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435:824–827
Alder A, Jamil M, Marzorati M, Bruno M, Vermathen M, Bigler P, Ghisla S, Bouwmeester H, Beyer P, Al-Babili S (2012) The path from beta-carotene to carlactone, a strigolactone-like plant hormone. Science 335:1348–1351
Arite T, Umehara M, Ishikawa S, Hanada A, Maekawa M, Yamaguchi S, Kyozuka J (2009) d14, a strigolactone-insensitive mutant of rice, shows an accelerated outgrowth of tillers. Plant Cell Physiol 50:1416–1424
Aroca R, Ruiz-Lozano JM, Zamarreno AM, Paz JA, Garcia-Mina JM, Pozo MJ, López-Ráez JA (2013) Arbuscular mycorrhizal symbiosis influences strigolactone production under salinity and alleviates salt stress in lettuce plants. J Plant Physiol 170:47–55
Beveridge CA, Kyozuka J (2009) New genes in the strigolactone-related shoot branching pathway. Curr Opin Plant Biol 13:34–39
Booker J, Auldridge M, Wills S, McCarty D, Klee H, Leyser O (2004) MAX3/CCD7 is a carotenoid cleavage dioxygenase required for the synthesis of a novel plant signaling molecule. Curr Biol 14:1232–1238
Booker J, Sieberer T, Wright W, Williamson L, Willett B, Stirnberg P, Turnbull CGN, Srinivasan M, Goddard P, Leyser O (2005) MAX1 encodes a cytochrome P450 family member that acts downstream of MAX3/4 to produce a carotenoid-derived branch-inhibiting hormone. Dev Cell 8:7
Bu Q, Lv T, Shen H, Luong P, Wang J, Wang Z, Huang Z, Xiao L, Engineer C, Kim TH, Schroeder JI, Huq E (2014) Regulation of drought tolerance by the F-box protein MAX2 in Arabidopsis. Plant Physiol 164:424–439
Cardoso C, Zhang Y, Jamil M, Hepworth J, Charnikhova T, Dimkpa SO, Meharg C, Wright MH, Liu J, Meng X, Wang Y, Li J, McCouch SR, Leyser O, Price AH, Bouwmeester HJ, Ruyter-Spira C (2014) Natural variation of rice strigolactone biosynthesis is associated with the deletion of two MAX1 orthologs. Proc Natl Acad Sci USA 111:2379–2384
Challis RJ, Hepworth J, Mouchel C, Waites R, Leyser O (2013) A role for more axillary growth 1 (MAX1) in evolutionary diversity in strigolactone signaling upstream of MAX2. Plant Physiol 161:1885–1902
Cheng X, Ruyter-Spira C, Bouwmeester H (2013) The interaction between strigolactones and other plant hormones in the regulation of plant development. Front Plant Sci 4:199
Deak KI, Malamy J (2005) Osmotic regulation of root system architecture. Plant J 43:17–28
Dodd IC, Ferguson BJ, Beveridge CA (2008) Apical wilting and petiole xylem vessel diameter of the rms2 branching mutant of pea are shoot controlled and independent of a long-distance signal regulating branching. Plant Cell Physiol 49:791–800
Drummond RS, Sheehan H, Simons JL, Martinez-Sanchez NM, Turner RM, Putterill J, Snowden KC (2012) The expression of petunia strigolactone pathway genes is altered as part of the endogenous developmental program. Front Plant Sci 2:115
Foo E, Davies NW (2011) Strigolactones promote nodulation in pea. Planta 234:1073–1081
Foo E, Yoneyama K, Hugill C, Quittenden LJ, Reid JB (2013a) Strigolactones: internal and external signals in plant symbioses? Plant Signal Behav 8:e23168
Foo E, Yoneyama K, Hugill CJ, Quittenden LJ, Reid JB (2013b) Strigolactones and the regulation of pea symbioses in response to nitrate and phosphate deficiency. Mol Plant 6:76–87
Gaiji N, Cardinale F, Prandi C, Bonfante P, Ranghino G (2012) The computational-based structure of Dwarf14 provides evidence for its role as potential strigolactone receptor in plants. BMC Res Notes 5:307
Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pagès V, Dun EA, Pillot JP, Letisse F, Matusova R, Danoun S, Portais JC, Bouwmeester H, Bécard G, Beveridge CA, Rameau C, Rochange SF (2008) Strigolactone inhibition of shoot branching. Nature 455:189–194
Ha CV, Leyva-Gonzalez MA, Osakabe Y, Tran UT, Nishiyama R, Watanabe Y, Tanaka M, Seki M, Yamaguchi S, Dong NV, Yamaguchi-Shinozaki K, Shinozaki K, Herrera-Estrella L, Tran LS (2014) Positive regulatory role of strigolactone in plant responses to drought and salt stress. Proc Natl Acad Sci USA 111:851–856
Hamiaux C, Drummond RS, Janssen BJ, Ledger SE, Cooney JM, Newcomb RD, Snowden KC (2012) DAD2 is an alpha/beta hydrolase likely to be involved in the perception of the plant branching hormone, strigolactone. Curr Biol 22:2032–2036
Handberg K, Stougaard J (1992) Lotus japonicus, an autogamous, diploid legume species for classical and molecular-genetics. Plant J 2:487–496
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. California agricultural experiment station, Circular 347. College of Agriculture, University of California, Berkeley
Iuchi S, Kobayashi M, Taji T, Naramoto M, Seki M, Kato T, Tabata S, Kakubari Y, Yamaguchi-Shinozaki K, Shinozaki K (2001) Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J 27:325–333
Jamil M, Charnikhova T, Cardoso C, Jamil T, Ueno K, Verstappen F, Asami T, Bouwmeester HJ (2011) Quantification of the relationship between strigolactones and Striga hermonthica infection in rice under varying levels of nitrogen and phosphorus. Weed Res 51:373–385
Jeschke WD, Peuke AD, Pate JS, Hartung W (1997) Transport, synthesis and catabolism of abscisic acid (ABA) in intact plants of castor bean (Ricinus communis L.) under phosphate deficiency and moderate salinity. J Exp Bot 48:1737–1747
Kagiyama M, Hirano Y, Mori T, Kim SY, Kyozuka J, Seto Y, Yamaguchi S, Hakoshima T (2013) Structures of D14 and D14L in the strigolactone and karrikin signaling pathways. Genes Cells 18:147–160
Kohlen W, Charnikhova T, Liu Q, Bours R, Domagalska MA, Beguerie S, Verstappen F, Leyser O, Bouwmeester H, Ruyter-Spira C (2011) Strigolactones are transported through the xylem and play a key role in shoot architectural response to phosphate deficiency in nonarbuscular mycorrhizal host Arabidopsis. Plant Physiol 155:974–987
Kolbert Z, Bartha B, Erdei L (2008) Osmotic stress- and indole-3-butyric acid-induced NO generation are partially distinct processes in root growth and development in Pisum sativum. Physiol Plant 133:406–416
Koltai H (2013) Strigolactones activate different hormonal pathways for regulation of root development in response to phosphate growth conditions. Ann Bot 112:409–415
Kretzschmar T, Kohlen W, Sasse J, Borghi L, Schlegel M, Bachelier JB, Reinhardt D, Bours R, Bouwmeester HJ, Martinoia E (2012) A petunia ABC protein controls strigolactone-dependent symbiotic signalling and branching. Nature 483:341–344
Lechat MM, Pouvreau JB, Peron T, Gauthier M, Montiel G, Veronesi C, Todoroki Y, Le Bizec B, Monteau F, Macherel D, Simier P, Thoiron S, Delavault P (2012) PrCYP707A1, an ABA catabolic gene, is a key component of Phelipanche ramosa seed germination in response to the strigolactone analogue GR24. J Exp Bot 63:5311–5322
Lin H, Wang R, Qian Q, Yan M, Meng X, Fu Z, Yan C, Jiang B, Su Z, Li J, Wang Y (2009) DWARF27, an iron-containing protein required for the biosynthesis of strigolactones, regulates rice tiller bud outgrowth. Plant Cell 21:1512–1525
Liu W, Kohlen W, Lillo A, Op den Camp R, Ivanov S, Hartog M, Limpens E, Jamil M, Smaczniak C, Kaufmann K, Yang WC, Hooiveld GJ, Charnikhova T, Bouwmeester HJ, Bisseling T, Geurts R (2011) Strigolactone biosynthesis in Medicago truncatula and rice requires the symbiotic GRAS-type transcription factors NSP1 and NSP2. Plant Cell 23:3853–3865
Liu J, Lovisolo C, Schubert A, Cardinale F (2013a) Signaling role of strigolactones at the interface between plants, (micro)organisms, and a changing environment. J Plant Interact 8:17–33
Liu J, Novero M, Charnikhova T, Ferrandino A, Schubert A, Ruyter-Spira C, Bonfante P, Lovisolo C, Bouwmeester HJ, Cardinale F (2013b) Carotenoid cleavage dioxygenase 7 modulates plant growth, reproduction, senescence, and determinate nodulation in the model legume Lotus japonicus. J Exp Bot 64:1967–1981
López-Ráez JA, Charnikhova T, Gómez-Roldán V, Matusova R, Kohlen W, De Vos R, Verstappen F, Puech-Pagès V, Bécard G, Mulder P, Bouwmeester H (2008) Tomato strigolactones are derived from carotenoids and their biosynthesis is promoted by phosphate starvation. New Phytol 178:863–874
López-Ráez JA, Kohlen W, Charnikhova T, Mulder P, Undas AK, Sergeant MJ, Verstappen F, Bugg TD, Thompson AJ, Ruyter-Spira C, Bouwmeester H (2010) Does abscisic acid affect strigolactone biosynthesis? New Phytol 187:343–354
Mashiguchi K, Sasaki E, Shimada Y, Nagae M, Ueno K, Nakano T, Yoneyama K, Suzuki Y, Asami T (2009) Feedback-regulation of strigolactone biosynthetic genes and strigolactone-regulated genes in Arabidopsis. Biosci Biotechnol Biochem 73:2460–2465
Mayzlish-Gati E, De-Cuyper C, Goormachtig S, Beeckman T, Vuylsteke M, Brewer PB, Beveridge CA, Yermiyahu U, Kaplan Y, Enzer Y, Wininger S, Resnick N, Cohen M, Kapulnik Y, Koltai H (2012) Strigolactones are involved in root response to low phosphate conditions in Arabidopsis. Plant Physiol 160:1329–1341
Mott KA, Sibbernsen ED, Shope JC (2008) The role of the mesophyll in stomatal responses to light and CO2. Plant Cell Environ 31:1299–1306
Nambara E, Marion-Poll A (2005) Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol 56:165–185
Osorio J, Osorio ML, Chaves MM, Pereira JS (1998) Water deficits are more important in delaying growth than in changing patterns of carbon allocation in Eucalyptus globulus. Tree Physiol 18:363–373
Qin X, Zeevaart JA (2002) Overexpression of a 9-cis-epoxycarotenoid dioxygenase gene in Nicotiana plumbaginifolia increases abscisic acid and phaseic acid levels and enhances drought tolerance. Plant Physiol 128:544–551
Radin J (1984) Stomatal responses to water stress and to abscisic acid in phosphorus-deficient cotton plants. Plant Physiol 76:392–394
Ruyter-Spira C, Kohlen W, Charnikhova T, van Zeijl A, van Bezouwen L, de Ruijter N, Cardoso C, López-Ráez JA, Matusova R, Bours R, Verstappen F, Bouwmeester H (2011) Physiological effects of the synthetic strigolactone analog GR24 on root system architecture in Arabidopsis: another belowground role for strigolactones? Plant Physiol 155:721–734
Ruyter-Spira C, Al-Babili S, van der Krol S, Bouwmeester H (2013) The biology of strigolactones. Trends Plant Sci 18:72–83
Scaffidi A, Waters MT, Bond CS, Dixon KW, Smith SM, Ghisalberti EL, Flematti GR (2012) Exploring the molecular mechanism of karrikins and strigolactones. Bioorg Med Chem Lett 22:3743–3746
Scholander PF, Bradstreet ED, Hemmingsen EA, Hammel HT (1965) Sap pressure in vascular plants. Negative hydrostatic pressure can be measured in plants. Science 148:339–346
Seto Y, Sado A, Asami K, Hanada A, Umehara M, Akiyama K, Yamaguchi S (2014) Carlactone is an endogenous biosynthetic precursor for strigolactones. Proc Natl Acad Sci USA 111:1640–1645
Shen H, Zhu L, Bu QY, Huq E (2012) MAX2 affects multiple hormones to promote photomorphogenesis. Mol Plant 5:750–762
Smith SM, Waters MT (2012) Strigolactones: destruction-dependent perception? Curr Biol 22:R924–R927
Sorefan K, Booker J, Haurogne K, Goussot M, Bainbridge K, Foo E, Chatfield S, Ward S, Beveridge C, Rameau C, Leyser O (2003) MAX4 and RMS1 are orthologous dioxygenase-like genes that regulate shoot branching in Arabidopsis and pea. Genes Dev 17:1469–1474
Soto MJ, Fernández-Aparicio M, Castellanos-Morales V, García-Garrido JM, Ocampo JA, Delgado MJ, Vierheilig H (2010) First indications for the involvement of strigolactones on nodule formation in alfalfa (Medicago sativa). Soil Biol Biochem 42:383–385
Stirnberg P, Furner IJ, Leyser HMO (2007) MAX2 participates in an SCF complex which acts locally at the node to suppress shoot branching. Plant J 50:80–94
Sugimoto Y, Ueyama T (2008) Production of (+)-5-deoxystrigol by Lotus japonicus root culture. Phytochemistry 69:212–217
Tan BC, Schwartz SH, Zeevaart JA, McCarty DR (1997) Genetic control of abscisic acid biosynthesis in maize. Proc Natl Acad Sci USA 94:12235–12240
Toh S, Kamiya Y, Kawakami N, Nambara E, McCourt P, Tsuchiya Y (2012) Thermoinhibition uncovers a role for strigolactones in Arabidopsis seed germination. Plant Cell Physiol 53:107–117
Torres-Vera R, Garcia JM, Pozo MJ, López-Ráez JA (2013) Do strigolactones contribute to plant defence? Mol Plant Pathol 15:211–216
Tramontini S, Vitali M, Centioni L, Schubert A, Lovisolo C (2013) Rootstock control of scion response to water stress in grapevine. Environ Exp Bot 93:20–26
Tsuchiya Y, McCourt P (2009) Strigolactones: a new hormone with a past. Curr Opin Plant Biol 12:556–561
Umehara M, Hanada A, Magome H, Takeda-Kamiya N, Yamaguchi S (2010) Contribution of strigolactones to the inhibition of tiller bud outgrowth under phosphate deficiency in rice. Plant Cell Physiol 51:1118–1126
Vandemoortele JL, Kevers C, Billard JP, Gaspar T (2001) Osmotic pretreatment promotes axillary shooting from cauliflower curd pieces by acting through internal cytokinin level modifications. J Plant Physiol 158:221–225
Waters MT, Nelson DC, Scaffidi A, Flematti GR, Sun YK, Dixon KW, Smith SM (2012a) Specialisation within the DWARF14 protein family confers distinct responses to karrikins and strigolactones in Arabidopsis. Development 139:1285–1295
Waters MT, Scaffidi A, Flematti GR, Smith SM (2012b) Karrikins force a rethink of strigolactone mode of action. Plant Signal Behav 7:969–972
Xiao BZ, Chen X, Xiang CB, Tang N, Zhang QF, Xiong LZ (2009) Evaluation of seven function-known candidate genes for their effects on improving drought resistance of transgenic rice under field conditions. Mol Plant 2:73–83
Xie X, Yoneyama K (2010) The strigolactone story. Annu Rev Phytopathol 48:93–117
Xiong L, Schumaker KS, Zhu JK (2002) Cell signaling during cold, drought, and salt stress. Plant Cell 14(Suppl):S165–S183
Yokota K, Fukai E, Madsen LH, Jurkiewicz A, Rueda P, Radutoiu S, Held M, Hossain MS, Szczyglowski K, Morieri G, Oldroyd GE, Downie JA, Nielsen MW, Rusek AM, Sato S, Tabata S, James EK, Oyaizu H, Sandal N, Stougaard J (2009) Rearrangement of actin cytoskeleton mediates invasion of Lotus japonicus roots by Mesorhizobium loti. Plant Cell 21:267–284
Yoneyama K, Awad AA, Xie X, Takeuchi Y (2010) Strigolactones as germination stimulants for root parasitic plants. Plant Cell Physiol 51:1095–1103
Yoneyama K, Xie X, Kim HI, Kisugi T, Nomura T, Sekimoto H, Yokota T (2012) How do nitrogen and phosphorus deficiencies affect strigolactone production and exudation? Planta 235:1197–1207
Zhang Y, van Dijk ADJ, Scaffidi A, Flematti GR, Hofmann M, Charnikhova T, Verstappen F, Hepworth J, van der Krol S, Leyser O, Smith SM, Zwanenburg B, Al-Babili S, Ruyter-Spira C, Bouwmeester HJ (2014) Rice cytochrome P450 MAX1 homologs catalyze distinct steps in strigolactone biosynthesis. Nat Chem Biol 10:1028–1033
Zhao LH, Zhou XE, Wu ZS, Yi W, Xu Y, Li S, Xu TH, Liu Y, Chen RZ, Kovach A, Kang Y, Hou L, He Y, Xie C, Song W, Zhong D, Wang Y, Li J, Zhang C, Melcher K, Xu HE (2013) Crystal structures of two phytohormone signal-transducing alpha/beta hydrolases: karrikin-signaling KAI2 and strigolactone-signaling DWARF14. Cell Res 23:436–439
Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53:247–273
Acknowledgments
We would like to thank Jens Stougaard (Aarhus University, Denmark) for the generous supply of Gifu B-129 seeds, Binne Zwanenburg (Radboud University, Nijmegen, The Netherlands) who kindly supplied some of the GR24 used and Tadao Asami (Department of Applied Biological Chemistry, University of Tokyo, Tokyo, Japan) for providing synthetic 5-deoxystrigol and D6-5-deoxystrigol. The authors also wish to thank Paola Bonfante (University of Turin, Italy) for continuous support on the topic. The research was funded by the BioBITs Project (Piedmont Region, Converging Technologies call 2007) and the SLEPS Project (Compagnia di S. Paolo and University of Turin, call 2012) to FC, AS, CL and IV, and by the Netherlands Organization for Scientific Research (NWO; Vici grant, 865.06.002, and Equipment grant, 834.08.001) to HJB. JL was funded by the Chinese Scholarship Council (CSC, Grant No. 2008108168).
Conflict of interest
FC, IV and AS hold a share of StrigoLab S.r.l., which provided part of the GR24 used in the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
H. He, M. Vitali and I. Visentin contributed equally to the work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, J., He, H., Vitali, M. et al. Osmotic stress represses strigolactone biosynthesis in Lotus japonicus roots: exploring the interaction between strigolactones and ABA under abiotic stress. Planta 241, 1435–1451 (2015). https://doi.org/10.1007/s00425-015-2266-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-015-2266-8