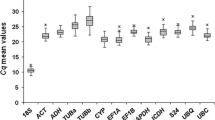
Abstract
Reverse transcription quantitative real-time PCR (RT-qPCR) is a sensitive technique for quantifying gene expression, but its success depends on the stability of the reference gene(s) used for data normalization. Only a few studies on validation of reference genes have been conducted in fruit trees and none in banana yet. In the present work, 20 candidate reference genes were selected, and their expression stability in 144 banana samples were evaluated and analyzed using two algorithms, geNorm and NormFinder. The samples consisted of eight sample sets collected under different experimental conditions, including various tissues, developmental stages, postharvest ripening, stresses (chilling, high temperature, and pathogen), and hormone treatments. Our results showed that different suitable reference gene(s) or combination of reference genes for normalization should be selected depending on the experimental conditions. The RPS2 and UBQ2 genes were validated as the most suitable reference genes across all tested samples. More importantly, our data further showed that the widely used reference genes, ACT and GAPDH, were not the most suitable reference genes in many banana sample sets. In addition, the expression of MaEBF1, a gene of interest that plays an important role in regulating fruit ripening, under different experimental conditions was used to further confirm the validated reference genes. Taken together, our results provide guidelines for reference gene(s) selection under different experimental conditions and a foundation for more accurate and widespread use of RT-qPCR in banana.





Similar content being viewed by others
Abbreviations
- ACT :
-
Actin
- APT :
-
Adenine phosphoribosyltransferase
- CAC :
-
Clathrin adaptor complexes medium
- CYP :
-
Cyclophilin
- DNAJ :
-
DnaJ-like protein
- EBF :
-
EIN3-binding F-box protein
- EF1α :
-
Elongation factor 1-alpha
- EIF5A :
-
Eukaryotic initiation factor 5A
- GAPDH :
-
Glyceraldehyde-3-phosphate dehydrogenase
- RAN :
-
GTP-binding nuclear protein
- RPL :
-
Ribosomal protein L
- RPS :
-
Ribosomal protein S
- RT-qPCR:
-
Reverse transcription quantitative real-time PCR
- SAMDC :
-
s-Adenosyl methionine decarboxylase
- TIP4I :
-
TIP4I-like family protein
- TUA :
-
Alpha-tubulin
- UBCE :
-
Ubiquitin conjugating enzyme
- UBQ :
-
Ubiquitin
References
An FY, Zhao Q, Ji YS, Li WY, Jiang ZQ, Yu XC, Zhang C, Han Y, He WR, Liu YD, Zhang SQ, Ecker JR, Guo HW (2010) Ethylene-induced stabilization of ETHYLENE INSENSITIVE3 and EIN3-LIKE1 is mediated by proteasomal degradation of EIN3 binding F-Box 1 and 2 that requires EIN2 in Arabidopsis. Plant Cell 22:2384–2401
Andersen CL, Jensen JL, Orntoft TF (2004) Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res 64:5245–5250
Artico S, Nardeli SM, Brilhante O, Grossi-de-Sa MF, Alves-Ferreira M (2010) Identification and evaluation of new reference genes in Gossypium hirsutum for accurate normalization of real-time quantitative RT-PCR data. BMC Plant Biol 10:49
Barsalobres-Cavallari CF, Severino FE, Maluf MP, Maia IG (2009) Identification of suitable internal control genes for expression studies in Coffea arabica under different experimental conditions. BMC Mol Biol 10:1
Brunner AM, Yakovlev IA, Strauss SH (2004) Validating internal controls for quantitative plant gene expression studies. BMC Plant Biol 4:14
Bustin SA (2002) Quantification of mRNA using real-time reverse transcription PCR RT-PCR: trends and problems. Mol Endocrinol 29:23–29
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622
Chen JY, He LH, Jiang YM, Wang Y, Joyce DC, Ji ZL, Lu WJ (2008) Role of phenylalanine ammonia-lyase in heat pretreatment-induced chilling tolerance in banana fruit. Physiol Plant 132:318–328
Chuaqui RF, Bonner RF, Best CJM, Gillespie JW, Flaig MJ, Hewitt SM, Phillips JL, Krizman DB, Tangrea MA, Ahram M, Linehan WM, Knezevic V, Emmert-Buck MR (2002) Post-analysis follow-up and validation of microarray experiments. Nat Genet 32:509–514
Cruz F, Kalaoun S, Nobile P, Colombo C, Almeida J, Barros L, Romano E, Grossi-de-Sá MF, Vaslin M, Alves-Ferreira M (2009) Evaluation of coffee reference genes for relative expression studies by quantitative real-time RT-PCR. Mol Breeding 23:607–616
Czechowski TSM, Altmann T, Udvardi MK, Scheible WR (2005) Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol 139:5–17
Derveaux S, Vandesompele J, Hellemans J (2010) How to do successful gene expression analysis using real-time PCR. Methods 50:227–230
Die JV, Román B, Nadal S, González-Verdejo CI (2010) Evaluation of candidate reference genes for expression studies in Pisum sativum under different experimental conditions. Planta 232:145–153
Dombrowski J, Martin R (2009) Evaluation of reference genes for quantitative RT-PCR in Lolium temulentum under abiotic stress. Plant Sci 176:390–396
Elitzur T, Vrebalov J, Giovannoni JJ, Goldschmidt EE, Friedman H (2010) The regulation of MADS-box gene expression during ripening of banana and their regulatory interaction with ethylene. J Exp Bot 61:1523–1535
Expósito-Rodríguez M, Borges A, Borges-Pérez A, Pérez J (2008) Selection of internal control genes for quantitative real-time RT-PCR studies during tomato development process. BMC Plant Biol 8:131
Gutierrez L, Mauriat M, Guénin S, Pelloux J, Lefebvre JF, Louvet R, Rusterucci C, Moritz T, Guerineau F, Bellini C, Van Wuytswinkel O (2008) The lack of a systematic validation of reference genes: a serious pitfall undervalued in reverse transcription polymerase chain reaction (RT-PCR) analysis in plants. Plant Biotechnol J 6:609–618
Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J (2007) qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol 8:R19
Hong SY, Seo PJ, Yang MS, Xiang F, Park CM (2008) Exploring valid reference genes for gene expression studies in Brachypodium distacyon by real-time PCR. BMC Plant Biol 8:112
Hu R, Fan C, Li H, Zhang Q, Fu Y-F (2009) Evaluation of putative reference genes for gene expression normalization in soybean by quantitative real-time RT-PCR. BMC Mol Biol 10:93
Huggett J, Dheda K, Bustin S, Zumla A (2005) Real-time RT-PCR normalization, strategies and considerations. Genes Immun 6:279–284
Inaba A, Liu X, Yokotani N, Yamane M, Lu WJ, Nakano R, Kubo Y (2007) Differential feedback regulation of ethylene biosynthesis in pulp and peel tissues of banana fruit. J Exp Bot 58:1047–1057
Iskandar HM, Simpson RS, Casu RE, Bonnet GD, Maclean DJ, Manners JM (2004) Comparison of reference genes for quantitative real-time polymerase chain reaction analysis of gene in sugarcane. Plant Mol Biol Rep 22:325–337
Jain M (2009) Genome-wide identification of novel internal control genes for normalization of gene expression during various stages of development in rice. Plant Sci 176:702–706
Jain M, Nijhawan A, Tyagi AK, Khurana JP (2006) Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real-time PCR. Biochem Biophys Res Commun 345:646–651
Jian B, Liu B, Bi Y, Hou W, Wu C, Han T (2008) Validation of internal control for gene expression study in soybean by quantitative real-time PCR. BMC Mol Biol 9:59
Kim B, Nam H, Kim S, Chang YJ (2003) Normalization of reverse transcription quantitative-PCR with housekeeping genes in rice. Biotechnol Lett 25:1869–1872
Le Bail A, Dittami S, de Franco PO, Rousvoal S, Cock M, Tonon T, Charrier B (2008) Normalisation genes for expression analyses in the brown alga model Ectocarpus siliculosus. BMC Mol Biol 9:75
Lee JM, Roche JR, Donaghy DJ, Thrush A, Sathish P (2010) Validation of reference genes for quantitative RT-PCR studies of gene expression in perennial ryegrass (Lolium perenne L.). BMC Mol Biol 11:8
Libault M, Thibivilliers S, Bilgin DD, Radwan O, Benitez M, Clough SJ, Stacey G (2008) Identification of four soybean reference genes for gene expression normalization. Plant Genome 1:44–54
Lin YL, Lai ZX (2010) Reference gene selection for qPCR analysis during somatic embryogenesis in longan tree. Plant Sci 178:359–365
Ma BC, Tang WL, Ma LY, Li LL, Zhang LB, Zhu SJ (2009) The role of chitinase gene expression in the defense of harvested banana against anthracnose disease. J Amer Soc Hort Sci 134:379–386
Mallona I, Lischewski S, Weiss J, Hause B, Egea-Cortines M (2010) Validation of reference genes for quantitative real-time PCR during leaf and flower development in Petunia hybrida. BMC Plant Biol 10:4
Maroufi A, Bockstaele EV, Loose MD (2010) Validation of reference genes for gene expression analysis in chicory (Cichorium intybus) using quantitative real-time PCR. BMC Mol Biol 11:15
Martin RC, Hollenbeck VG, Dombrowski JE (2008) Evaluation of reference genes for quantitative RT-PCR in Lolium perenne. Crop Sci 48:1881–1887
Mbéguié-A-Mbéguié D, Hubert O, Fils-Lycaon B, Chillet M, Baurens FC (2008) EIN3-like gene expression during fruit ripening of Cavendish banana (Musa acuminata cv. Grande naine). Physiol Plant 133:435–448
Mbéguié-A-Mbéguié D, Hubert O, Baurens FC, Matsumoto T, Chillet M, Fils-Lycaon B, Sidibé-Bocs S (2009) Expression patterns of cell wall-modifying genes from banana during fruit ripening and in relationship with finger drop. J Exp Bot 60:2021–2034
Mukesh J, Aashima N, Akhilesh KT, Jitendra PK (2006) Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real-time PCR. Biochem Biophys Res Commun 345:646–651
Nicot N, Hausman JF, Hoffmann L, Evers D (2005) Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J Exp Bot 56:2907–2914
Nolan T, Hands RE, Bustin SA (2006) Quantification of mRNA using real-time RTPCR. Nat Protocols 1:1559–1582
Paolacci AR, Tanzarella OA, Porceddu EP, Ciaffi M (2009) Identification and validation of reference genes for quantitative RT-PCR normalization in wheat. BMC Mol Biol 10:11
Pfaffl MWTA, Prgomet C, Neuvians TP (2004) Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper-Excel-based tool using pair-wise correlations. Biotechnol Lett 26:509–515
Radonic A, Thulke S, Mackay IM, Landt O, Siegert W, Nitsche A (2004) Guideline to reference gene selection for quantitative real-time PCR. Biochem Biophys Res Commun 23:856–862
Reid KE, Olsson N, Schlosser J, Peng F, Lund ST (2006) An optimized grapevine RNA isolation procedure and statistical determination of reference genes for real-time RT-PCR during berry development. BMC Plant Biol 6:27
Remans T, Smeets K, Opdenakker K, Mathijsen D, Vangronsveld J, Cuypers A (2008) Normalisation of real-time RT-PCR gene expression measurements in Arabidopsis thaliana exposed to increased metal concentrations. Planta 227:1343–1349
Roy CS, Roy S, Singh SK, Sengupta DN (2010) Molecular characterization and differential expression of beta-1, 3-glucanase during ripening in banana fruit in response to ethylene, auxin, ABA, wounding, cold and light-dark cycles. Plant Cell Rep 29:813–828
Schmidt GW, Delaney SK (2010) Stable internal reference genes for normalization of real-time RT-PCR in tobacco (Nicotiana tabacum) during development and abiotic stress. Mol Genet Genomics 283:233–241
Silveira ED, Alves-Ferreira M, Guimarães LA, Rodrigues da Silva F, Carneiro V (2009) Selection of reference genes for quantitative real-time PCR expression studies in the apomictic and sexual grass Brachiaria brizantha. BMC Plant Biol 9:84
Tang WL, Zhu SJ, Li LL, Liu DJ, Irving DE (2010) Differential expressions of PR1 and chitinase genes in harvested bananas during ripening, and in response to ethephon, benzothiadizole and methyl jasmonate. Postharvest Biol Technol 57:86–91
Tong Z, Gao Z, Wang F, Zhou J, Zhang Z (2009) Selection of reliable reference genes for gene expression studies in peach using realtime PCR. BMC Mol Biol 10:71
Tu L, Zhang X, Liu D, Jin S, Cao J, Zhu L, Deng F, Tan J, Zhang C (2007) Suitable internal control genes for qRT-PCR normalization in cotton fiber development and somatic embryogenesis. Chin Sci Bull 52:3110–3117
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalisation of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:1–11
Wan CY, Wilkins TA (1994) A modified hot borate method significantly enhances the yield of high-quality RNA from cotton (Gossypium hirsutum L.). Anal Biochem 223:7–12
Wan HJ, Zhao ZG, Qian CT, Sui YH, Malik AA, Chen JF (2010) Selection of appropriate reference genes for gene expression studies by quantitative real-time polymerase chain reaction in cucumber. Anal Biochem 399:257–261
Wang Y, Wu J, Xu BY, Liu JH, Zhang JB, Jia CH, Jin ZQ (2010) Cloning of an ADP-ribosylation factor gene from banana (Musa acuminata) and its expression patterns in postharvest ripening fruit. J Plant Physiol 167:989–995
Wong ML, Medrano JF (2005) Real-time PCR for mRNA quantitation. BioTechniques 39:75–85
Yang YG, Wu Y, Pirrello J, Regad F, Bouzayen M, Deng W, Li ZG (2009) Silencing Sl-EBF1 and Sl-EBF2 expression causes constitutive ethylene response phenotype, accelerated plant senescence, and fruit ripening in tomato. J Exp Bot 61:697–708
Acknowledgments
We thank Dr. Yuhai Cui (Agriculture and Agri-Food Canada, Southern Crop Protection and Food Research Centre, London, Canada) for his helpful discussion and revising. We also thank the three reviewers for their helpful suggestions. This study was financially supported by the National Natural Science Foundation of China (Grant Nos. 30800772; 30972068) and Modern Agro-industry Technology Research System (Grant No. nycytx-33).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, L., Zhong, Hy., Kuang, Jf. et al. Validation of reference genes for RT-qPCR studies of gene expression in banana fruit under different experimental conditions. Planta 234, 377–390 (2011). https://doi.org/10.1007/s00425-011-1410-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-011-1410-3