Abstract
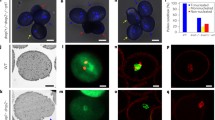
The promoter sequence of sperm-expressed gene, PzIPT isolated from the Svn (sperm associated with the vegetative nucleus) of Plumbago zeylanica, was fused to a green fluorescent protein (GFP) reporter sequence and transformed into Arabidopsis thaliana to better visualize the live behavior of angiosperm sperm cells. Angiosperm sperm cells are not independently motile, migrating in a unique cell-within-a-cell configuration within the pollen tube. Sperm cells occur in association with the vegetative nucleus forming a male germ unit (MGU). In Arabidopsis, GFP was expressed equally in both sperm cells and was observed using a spinning disk confocal microscope, which allowed long duration observation of cells without bleaching or visible laser radiation damage. Pollen activation is reflected by conspicuous movement of sperm and pollen cytoplasm. Upon pollen germination, sperm cells enter the forming tube and become oriented, typically with a sperm cytoplasmic projection leading the sperm cells in the MGU, which remains intact throughout normal pollen tube elongation. Maturational changes, including vacuolization, general rounding and entry into G2, were observed during in vitro culture. When MGUs were experimentally disrupted by mild temperature elevation, sperm cells no longer tracked the growth of the tube and separated from the MGU, providing critical direct evidence that the MGU is a functional unit required for sperm transmission.




Similar content being viewed by others
Abbreviations
- CCDs:
-
Charge-coupled devices
- GFP:
-
Green fluorescent protein
- GUS:
-
β-Glucuronidase
- IPT:
-
Isopentenyl transferase
- MGU:
-
Male germ unit
- Pz:
-
Plumbago zeylanica
References
Becker D (1990) Binary vectors which allow the exchange of plant selectable markers and reporter genes. Nucl Acids Res 18:203
Berger F, Hamamura Y, Ingouff M, Higashiyama T (2008) Double fertilization: caught in the act. Trends Plant Sci 13:437–443
Boavida LC, McCormick S (2007) Temperature as a determinant factor for increased and reproducible in vitro pollen germination in Arabidopsis thaliana. Plant J 52:570–582
Borges F, Gomes G, Gardner R, Moreno N, McCormick S, Feijo JA, Becker JD (2008) Comparative transcriptomics of Arabidopsis thaliana sperm cells. Plant Physiol 148:1168–1181
Cheung AY, Boavida LC, Aggarwal M, Wu H-M, Feijo JA (2010) The pollen tube journey in the pistil and imaging the in vivo process by two-photon microscopy. J Exp Bot 61:1907–1915
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Dumas C, Knox RB, McConchie CA, Russell SD (1984) Emerging physiological concepts in fertilization. What’s New Plant Physiol 15:17–20
Engel ML, Davis RH, McCormick S (2005) Green sperm. Identification of male gamete promoters in Arabidopsis. Plant Physiol 138:2124–2133
Friedman WE (1999) Expression of the cell cycle in sperm of Arabidopsis: implications for understanding patterns of gametogenesis and fertilization in plants and other eukaryotes. Development 126:1065–1075
Gou XP, Yuan T, Wei XP, Russell SD (2009) Gene expression in the dimorphic sperm cells of Plumbago zeylanica: transcript profiling, diversity, and relationship to cell type. Plant J 60:33–47
Hepler PK, Vidali L, Cheung AY (2001) Polarized cell growth in higher plants. Annu Rev Cell Devel Biol 17:159–187
Higashiyama T, Hamamura Y (2008) Gametophytic pollen tube guidance. Sex Plant Reprod 21:17–26
Kliwer I, Dresselhaus T (2010) Establishment of the male germline and sperm cell movement during pollen germination and tube growth in maize. Plant Signal Behav 5:1–5
Ma H (2005) Molecular genetic analyses of microsporogenesis and microgametogenesis in flowering plants. Annu Rev Plant Biol 56:393–434
Maheshwari P (1950) An introduction to the embryogenesis of angiosperms. McGraw-Hill, New York
Mogensen HL (1992) The male germ unit: concept, composition and signification. Intl Rev Cytol 140:129–147
Qin Y, Leydon AR, Manziello A, Pandey R, Mount D, Denic S, Vasic B, Johnson MA, Palanivelu R (2009) Penetration of the stigma and style elicits a novel transcriptome in pollen tubes, pointing to genes critical for growth in a pistil. PLoS Genet 5:e1000621
Russell SD (1992) Double fertilization. Intl Rev Cytol 140:357–388
Russell SD, Cass DD (1981) Ultrastructure of the sperms of Plumbago zeylanica. l. Cytology and association with the vegetative nucleus. Protoplasma 107:85–107
Russell SD, Rougier M, Dumas C (1990) Organization of the early post-fertilization megagametophyte of Populus deltoides. Ultrastructure and implications for male cytoplasmic transmission. Protoplasma 155:153–165
Singh M, Bhalla P, Russell S (2008) Molecular repertoire of flowering plant male germ cells. Sex Plant Reprod 21:27–36
Wan SQ, Yuan TBS, Wallace L, Russell SD, Luo Y (2002) Response of an allergenic species, Ambrosia psilostachya (Asteraceae), to experimental warming and clipping: Implications for public health. Am J Bot 89:1843–1846
Weigel D, Ahn JH, Blázquez MA, Borevitz JO, Christensen SK, Fankhauser C, Ferrándiz C, Kardailsky I, Malancharuvil EJ, Neff MM et al (2000) Activation tagging in Arabidopsis. Plant Physiol 122:1003–1013
Xu H, Swoboda I, Bhalla PL, Singh MB (1999) Male gametic cell-specific gene expression in flowering plants. Proc Natl Acad Sci USA 96:2554–2558
Yu HS, Huang BQ, Russell SD (1994) Transmission of male cytoplasm during fertilization in Nicotiana tabacum. Sex Plant Reprod 7:313–323
Acknowledgments
We thank Drs. Jia Li (Lanzhou University, China), Xiaoping Wei (University of Oklahoma), Mohan Singh and Prem Bhalla (University of Melbourne, Australia) for helpful advice and discussion. We are grateful for the help of Men Yongfan, Peking University, with Matlab applications. Research support was provided by University of Oklahoma, Samuel Roberts Noble Foundation, and the National Science Foundation Major Research Instrumentation grant (DBI-0722635), under which the spinning disk confocal microscope was purchased.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Movie 1 Spinning disk confocal microscopic images of growing pollen tube containing GFP-labeled sperm cells using interference contrast microscopy integrated with fluorescence imaging. Image interval: ≈7 seconds. This video displays a representative group of pollen grains showing migration of cytoplasmic organelles and GFP labeled sperm cytoplasm in activated pollen (some actively germinating), as well as some pollen that display no cytoplasmic or sperm movement. Some unactivated pollen grains contain GFP fluorescent sperm cells, indicating that failure mechanism of pollen to germinate may occur after sperm maturation and thus quite late in development. Occurrence of sperm movement is a useful (and particularly relevant) criterion for assessing male fertility. (MPEG 3054 kb)
Supplementary Movie 2. Spinning disk confocal microscopy showing sperm cells soon after entry into the pollen tube with initially erratic directionality until axial directionality is established. As cytoplasmic projection may lead or follow and may even rotate within the tube, it is evident that multiple foci of translocation are involved in sperm cell transport. Note that the cytoplasmic projection linkage maintains association with the tube nucleus but does not appear to move the sperm cells. (MPEG 4014 kb)
Supplementary Movie 3. Spinning disk confocal microscopy showing cropped processed images of a linked pair of sperm cells showing projection and dynamism of sperm during their descent in the pollen tube. The cellular projection of one sperm cell displays movement often independent of the overall momentum of the sperm cells. It is particularly noteworthy that the cellular projection appears to change length and its projection often appears slack and not under tension. The projection periodically appears to retreat, while the rest of the sperm cell with the other linked sperm cell proceed forward. (MPEG 1786 kb)
Supplementary Movie 4. Spinning disk confocal microscopy of paired sperm cells 5 h after germination, representing maturing sperm cells late in the progamic stage. Cells appear to become more elongated, accumulate vacuoles and often became flattened. Corresponding to the time period at which a rapidly elongating pollen tube approaches the ovary, this image reflects some changes reported in the literature, as well as indicating subtle changes in sperm cell shape that appear to accompany this late stage. (MPEG 2224 kb)
Supplementary Movie 5. Spinning disk confocal microscopy of effects of mild thermal treatment on sperm cells in germinated pollen tubes in which the two MGUs shown in Fig. 4 have become dissociated, whereas other MGUs may not necessarily display this defect. Dissociated sperm cells may fail to enter the pollen tube, or may separate within the tube and fail to maintain sufficient forward movement to track the growing tip. Dissociated sperm cells in the pollen tube typically move randomly relative to each other. Restoring elongating pollen tubes to normal temperatures does not restore normal sperm cell associations. (MPEG 3486 kb)
Rights and permissions
About this article
Cite this article
Ge, L., Gou, X., Yuan, T. et al. Migration of sperm cells during pollen tube elongation in Arabidopsis thaliana: behavior during transport, maturation and upon dissociation of male germ unit associations. Planta 233, 325–332 (2011). https://doi.org/10.1007/s00425-010-1305-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-010-1305-8