Abstract
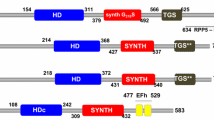
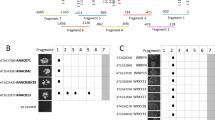
The stringent response is one of the most important regulatory systems in bacteria for adaptation to environmental stresses such as amino acid starvation. This response is mediated by an unusual nucleotide, guanosine 3′,5′-bis(pyrophosphate) (ppGpp), the levels of which are controlled by two enzymes, RelA and SpoT. ppGpp dramatically influences a broad range of physiological activities in bacterial cells, including transcription and translation as well as the enzymatic activities of several metabolic pathways. Recently, a growing number of RelA/SpoT homologues, designated RSH, have been identified in plants. Arabidopsis has four RSHs (AtRSH1, AtRSH2, AtRSH3 and AtCRSH). In order to reveal the specific roles of the individual Arabidopsis RSHs, we have analyzed the ppGpp synthase activities of the four RSHs and characterized the expression pattern of the RSH genes. Results from the present and previous studies indicate that all four RSH proteins are targeted into plastids. Complementation analysis of E. coli mutants revealed that AtRSH2, AtRSH3 and AtCRSH, but not AtRSH1, have ppGpp synthetase activity. Promoter analysis using reporter gene fusions indicated that the four Arabidopsis RSH genes are expressed in green tissues and flowers, suggesting the involvement of RSH functions in chloroplast development and reproduction. We also observed that all RSH transcripts exhibit a diurnal rhythm, and that induction of AtRSH1 and AtRSH2 transcripts are responsive to several environmental stresses. These results suggest that expression of the four RSH genes is coordinately regulated and that the ppGpp-dependent plastid stringent response has certain roles in the physiology of higher plants.






Similar content being viewed by others
Abbreviations
- ABA:
-
Abscisic acid
- CRSH:
-
Ca2+-activated RelA/SpoT homolog
- GFP:
-
Green fluorescent protein
- GUS:
-
β-Glucuronidase
- IPTG:
-
Isopropyl-β-d-thiogalactopyranoside
- JA:
-
Jasmonic acid
- MeJA:
-
Methyl jasmonate
- OPDA:
-
12-Oxo-phytodienoic acid
- ORGs:
-
OPDA-specific response genes
- ppGpp:
-
Guanosine 3′,5′-bis(pyrophosphate)
- RSH:
-
RelA/SpoT homolog
References
Aravind L, Koonin EV (1998) The HD domain defines a new superfamily of metal-dependent phosphohydrolases. Trends Biochem Sci 23:469–472
Artsimovitch I, Patlan V, Sekine S, Vassylyeva MN, Hosaka T, Ochi K, Yokoyama S, Vassylyev DG (2004) Structural basis for transcription regulation by alarmone ppGpp. Cell 117:299–310
Balbi V, Devoto A (2007) Jasmonate signaling network in Arabidopsis thaliana: crucial regulatory nodes and new physiological scenarios. New Phytol 177:301–318
Bechtold N, Pelletier G (1998) In planta Agrobacterium-mediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. Methods Mol Biol 82:259–266
Bechtold N, Ellis J, Pelletier G (1993) In planta Agrobacterium mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C R Acad Sci Paris Life Sci 316:1194–1199
Cashel M, Gentry DR, Hernandez VJ, Vinella D (1996) The stringent response. In: Neidhart FC, Curtiss RIII, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE (eds) Escherichia coli and Salmonella: cellular and molecular biology, 2nd edn. AMS Press, Washington DC, pp 1458–1496
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159
Chui W, Niwa Y, Zeng W, Hirano T, Kobayashi H, Sheen J (1996) Engineering GFP as vital reporter in plants. Curr Biol 6:325–330
Givens RM, Lin M, Taylor DJ, Mechold U, Berry JO, Hernandez VJ (2004) Inducible expression, enzymatic activity, and origin of higher plant homologues of bacterial RelA/SpoT stress proteins in Nicotiana tabacum. J Biol Chem 279:7495–7504
Gray MW (1993) Origin and evolution of organelle genomes. Curr Opin Genet Dev 3:884–890
Hirayama T, Shinozaki K (2007) Perception and transduction of abscisic acid signals: keys to the function of the versatile plant hormone ABA. Trends Plant Sci 12:343–351
Hou Z, Cashel M, Fromm HJ, Honzatko RB (1999) Effectors of the stringent response target the active site of Escherichia coli adenylosuccinate synthetase. J Biol Chem 274:17505–17510
Kirk JTO, Tilney-Bassett RAE (1978) Proplastids, etioplasts, amyloplasts, chromoplasts and other plastids. In: Kirk JTO, Tilney-Bassett RAE (eds) The plastids: their chemistry, structure, growth and inheritance, 2nd edn. Elsevier/North Holland Biomedical Press, Amsterdam, pp 217–239
Masuda S, Bauer CE (2004) Null mutation of HvrA compensates for loss of an essential relA/spoT-like gene in Rhodobacter capsulatus. J Bacteriol 186:235–239
Masuda S, Mizusawa K, Narisawa T, Tozawa Y, Ohta H, Takamiya K (2008) The bacterial stringent response, conserved in chloroplasts, controls plant fertilization. Plant Cell Physiol 49:135–141
Sanders PM, Bui AQ, Weterings K, McIntire KN, Hsu Y, Lee PY, Truong MT, Beals T, Goldberg RB (1999) Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Sex Plant Reprod 11:297–322
Smyth DR, Bowman JL, Meyerowitz EM (1990) Early flower development in Arabidopsis. Plant Cell 2:755–767
Takahashi K, Kasai K, Ochi K (2004) Identification of the bacterial alarmone guanosine 5′-diphosphate 3′-diphosphate (ppGpp) in plants. Proc Natl Acad Sci USA 101:4320–4324
Taki N, Sasaki-Sekimoto Y, Obayashi T, Kikuta A, Kobayashi K, Ainai T, Yagi K, Sakurai N, Suzuki H, Masuda T, Takamiya K, Shibata D, Kobayashi Y, Ohta H (2005) 12-oxo-phytodienoic acid triggers expression of a distinct set of genes and plays a role in wound-induced gene expression in Arabidopsis. Plant Physiol 139:1268–1283
Tozawa Y, Nozawa A, Kanno T, Narisawa T, Masuda S, Kasai K, Nanamiya H (2007) Calcium-activated (p)ppGpp synthetase in chloroplasts of land plants. J Biol Chem 282:35536–35545
Uzan M, Danchin A (1976) A rapid test for the relA mutation in E. coli. Biochem Biophys Res Commun 69:751–758
van der Biezen EA, Sun J, Coleman MJ, Bibb MJ, Jones JD (2000) Arabidopsis RelA/SpoT homologs implicate (p)ppGpp in plant signaling. Proc Natl Acad Sci USA 97:3747–3752
Verslues PE, Zhu J-K (2007) New developments in abscisic acid perception and metabolism. Curr Opin Plant Biol 10:447–452
Wang JD, Sanders GM, Grossman AD (2007) Nutritional control of elongation of DNA replication by (p)ppGpp. Cell 128:865–875
Wendrich TM, Marahiel MA (1997) Cloning and characterization of a relA/spoT homologue from Bacillus subtilis. Mol Microbiol 26:65–79
Xiao H, Kalman M, Ikehara K, Zemel S, Glaser G, Cashel M (1991) Residual guanosine 3′,5′-bispyrophosphate synthetic activity of relA null mutants canbe eliminated by spoT null mutations. J Biol Chem 266:5980–5990
Acknowledgments
The authors dedicate this paper to the late Professor Ken-ichiro Takamiya (Tokyo Institute of Technology, Yokohama, Japan). We thank Yuzuru Tozawa (Ehime University, Matsuyama, Japan), Hiroshi Shimada and Mie Shimojima (Tokyo Institute of Technology) for helpful suggestions during the study. This work was supported by the Research Foundation for Opto-Science and Technology (to S. Masuda); and the Ministry of Education, Culture, Science and Technology of Japan (to S. Masuda and H. Ohta).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mizusawa, K., Masuda, S. & Ohta, H. Expression profiling of four RelA/SpoT-like proteins, homologues of bacterial stringent factors, in Arabidopsis thaliana . Planta 228, 553–562 (2008). https://doi.org/10.1007/s00425-008-0758-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-008-0758-5