Abstract
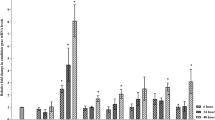
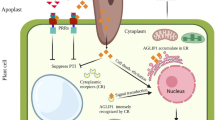
Pseudomonas cichorii causes necrotic leaf spots (NLS), while Pseudomonas syringae pv. tabaci induces a hypersensitive response (HR) in eggplant. P. cichorii induced cell death at 9 h after inoculation (HAI), reaching a maximum of around 24–30 HAI. On the other hand, cell death was induced 6 HAI with P. syringae pv. tabaci, reaching a maximum of around 12–18 HAI. Superoxide generation was observed in eggplant inoculated with both bacteria. DNA fragmentation, cytochrome c release into the cytosol and expression of defense-related genes such as PR-1 and hsr203J was also induced by inoculation with both bacteria, but these plant reactions were more rapidly induced in eggplant inoculated with P. syringae pv. tabaci rather than those with P. cichorii. Lipid peroxidation and induction of lipoxygenase (LOX) was drastically induced in eggplant inoculated with P. syringae pv. tabaci compared to P. cichorii-inoculated eggplant. Pharmacological studies showed that induction of the cell death, and the NLS or the HR in response to both bacteria was commonly associated with de novo protein synthesis, reactive oxygen species and caspase III-like protease. Interestingly, involvement of lipid peroxidation, LOX, serine protease, and DNase differed between induction of NLS and HR. These results suggest that programmed cell death might be closely associated not only with the HR but also NLS. However, there may be differences not only in the induction kinetics and level of plant responses but also in the infection-related responses between HR and NLS.














Similar content being viewed by others
Abbreviations
- CFU:
-
Colony forming units
- DIG:
-
Digoxigenin
- HR:
-
Hypersensitive response
- LOX:
-
Lipoxygenase
- PAGE:
-
Polyacrylamide electrophoresis
- PCD:
-
Programmed cell death
- PR:
-
Pathogenesis-related
- PVDF:
-
Polyvinylidene difluoride
- ROS:
-
Reactive oxygen species
References
Able AJ (2003) Role of reactive oxygen species in the response of barley to necrotrophic pathogens. Protoplasma 221:137–143
Adam A, Farkas T, Somlyai G, Hevesi M, Kiraly Z (1989) Consequence of O -2 generation during a bacterially induced hypersensitive reaction in tobacco: deterioration of membrane lipids. Physiol Mol Plant Pathol 34:13–26
Altschul SF, Gish W, Miller W, Liptman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Abel AJ, Guest DI, Sutherland MW (1998) Use of new tetrazoliun-based assay to study the production of superoxide radicals by tobacco cell cultures challenged with virulent zoospores of Phytophthora parasitica var nicotianae. Plant Physiol 117:491–499
Baker CJ, Mock NM (1994) An improved method for monitoring cell death in cell suspension and leaf disc assays using evans blue. Plant Cell Tissue Organ Cult 39:7–12
Basu S (2003) Carbon tetrachloride-induced lipid peroxidation: eicosanoid formation and their regulation by antioxidant nutrients. Toxicology 189:113–127
Beers EP, Woffenden BJ, Zhao CS (2000) Plant proteolytic enzymes: possible roles during programmed cell death. Plant Mol Biol 44:399–415
Brown PH, Ho T-HD (1986) Barley aleurone layers secrete a nuclease in response to gibberellic acid. Plant Physiol 82:801–806
Buonaurio R, Servili M (1999) Involvement of lipoxygenase, lipoxygenase pathway volatiles, and lipid peroxidation during the hypersensitive reaction of pepper leaves to Xanthomonas campestris pv. vesicatoria. Physiol Mol Plant Pathol 54:155–169
Casano LM, Desimone M, Trippi VS (1989) Proteolytic activity at alkaline pH in oat leaves, isolation of an aminopeptidase. Plant Physiol 91:1414–1418
Casolo V, Petrussa E, Krajnakova J, Macrı F, Vianello A (2005) Involvement of the mitochondrial K+ ATP channel in H2O2- or NO-induced programmed death of soybean suspension cell cultures. J Exp Bot 56:997–1006
Danon A, Delorme V, Mailhae N, Gallois P (2000) Plant programmed cell death: a common way to die. Plant Physiol Biochem 38:647–655
del Pozo O, Lam E (1998) Caspase and programmed cell death in the hypersensitive response of plants to pathogens. Curr Biol 8:1129–1132
del Pozo O, Lam E (2003) Expression of the baculovirus p35 protein in tobacco affects cell death progression and compromises N gene-mediated disease resistance response to Tobacco mosaic virus. Mol Plant Microbe Interact 16:485–494
del Pozo O, Pedley KF, Martin GB (2004) MAPKKKα is a positive regulator of cell death associated with both plant immunity and disease. EMBO J 23:3072–3082
Doke N, Miura Y, Sanchez LM, Park H-J, Noritake T, Yoshioka H, Kawakita K (1996) The oxidative burst protects plants against pathogen attack: mechanism and role as emergency signal for plant bio-defense- a review. Gene 179:45–51
D’Silva I, Poirier GG, Heath MC (1998) Activation of cysteine proteases in cowpea plants during the hypersensitive response-a form of programmed cell death. Exp Cell Res 245:389–399
Govrin EM, Levine A (2000) The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr Biol 10:751–757
Greenberg JT, Yao N (2004) The role and regulation of programmed cell death in plant–pathogen interactions. Cell Microbiol 6:201–211
Hatsugai N, Kuroyanagi M, Yamada K, Meshi T, Tsuda S, Kondo M, Nishimura M, Hara-Nishimura (2004) A plant vacuolar protease, VPE, mediates virus-induced hypersensitive cell death. Science 305:855–858
Heath MC (2000) Hypersensitive response-related death. Plant Mol Biol 44:321–334
Hoeberichts FA, Woltering EJ (2003) Multiple mediators of plant programmed cell death: interplay of conserved cell death mechanisms and plant-specific regulator. Bioessays 25:47–57
Jalloul A, Montillet JL, Assigbetse K, Agnel JP, Delannoy E, Triantaphylides C, Daniel JF, Marmey P, Geiger JP, Nicole1 M (2002) Lipid peroxidation in cotton: Xanthomonas interactions and the role of lipoxygenases during the hypersensitive reaction. Plant J l:1–12
Jones AM, Coimbra S, Fath A, Sottomayor M, Thomas H (2001) Programmed cell death assay for plants. Method Cell Biol 66:437–451
Keppler LD, Baker CJ (1989) O -2 -initiated lipid peroxidation in a bacteria-induced hypersensitive reaction in tobacco cells suspensions. Phytopathology 79:555–562
Keppler LD, Novacky A (1986) Involvement of membrane lipid peroxidation in the development of a bacterially induced hypersensitive reaction. Phytopathology 76:104–108
Kiba A, Tomiyama H, Takahashi H, Hamada H, Ohnishi K, Okuno T, Hikichi Y (2003a) Induction of resistance and expression of defense-related genes in tobacco leaves infiltrated with Ralstonia solanacearum. Plant Cell Physiol 44:287–295
Kiba A, Sangawa Y, Hojo H, Ohnishi K, Hikichi Y (2003b) Involvement of apoptotic cell death in development of bacterial rot disease caused by Pseudomonas cichorii. In: Tikhonovich I et al (ed) Biology of plant–microbe interactions. International society for molecular plant-microbe interactions, St. Paul, vol 4. pp 67–69
Kiba A, Sangawa Y, Ohnishi K, Yao N, Park P, Nakayashiki H, Tosa Y, Mayama S, Hikichi Y (2006) Induction of apoptotic cell death leads to the development of bacterial rot caused by Pseudomonas cichorii. Mol Plant Microbe Interact 19:112–122
Kromer G, Zamzami N, Susin SA (1997) Mitochondrial control of apoptosis. Immunol Today 18:44–51
Lam E, del Pozo O (2000) Caspase-like protease involvement in the control of plant cell death. Plant Mol Biol 44:417–428
Lam E, Kato N, Lawton M (2001) Programmed cell death, mitochondria and plant hypersensitive response. Nature 411:848–853
Lamb CJ, Ryals JA, Ward ER, Dixon RA (1992) Emerging strategies for enhancing crop resistance to microbial pathogens. Biotechnology 10:1436–1445
Lincoln JE, Richael C, Overduin B, Smith K, Bostock R, Gilchrist DG (2002) Expression of the antiapoptotic baculovirus p35 gene in tomato blocks programmed cell death and provides broad-spectrum resistance to disease. Proc Natl Acad Sci USA 99:15217–15221
Levine A, Tenhaken R, Dixon R, Lamb C (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79:583–593
May MJ, Hammond-Kosack KE, Jones JDG (1996) Involvement of reactive oxygen species, glutathione metabolism, and lipid peroxidation in the Cf-gene dependent defense response of tomato cotyledons induced by race-specific elicitor of a Cladosporium fulvum. Plant Physiol 110:1367–1379
Mittler R, Lam E (1995) Identification, characterization, and purification of a tobacco endonuclease activity induced upon hypersensitive response cell death. Plant Cell 7:1951–1962
Nagata S (2005) DNA degradation in development and programmed cell death. Annu Rev Immunol 23:853–875
Navarre DA, Wolpert TJ (1999) Victorin induction of an apoptotic/senescence-like response in oats. Plant Cell 11:237–249
Petit PX, Zamzami N, Vayssiere JL, Mignotte B, Kroemer G, Castedo M (1997) Implication of mitochondria in apoptosis. Mol Cell Biochem 174:185–188
Pontier D, Balague C, Roby D (1998) The hypersensitive response. A programmed cell death associated with plant resistance. C R Acad Sci III 321:721–734
Richael C, Lincoln JE, Bostock RM, Gilchrist DG (2001) Caspase inhibitors reduce symptom development and limit bacterial proliferation in susceptible plant tissues. Physiol Mol Plant Pathol 59:213–221
Rusterucci C, Stallaert V, Milat ML, Pugin A, Ricci P, Blein JP (1996) Relationship between active oxygen species, lipid peroxidation, necrosis, and phytoalexin production induced by elicitins in Nicotiana. Plant Physiol 111:885–891
Ryerson DE, Heath MC (1996) Cleavage of nuclear DNA into oligonucleosomal fragments during cell death induced by fungal infection or by abiotic treatments. Plant Cell 8:393–402
Saraste A, Pulkki K (2000) Morphologic and biochemical hallmarks of apoptosis. Cardiovasc Res 45:528–537
Sasabe M, Takeuchi K, Kamoun S, Ichinose Y, Govers F, Toyoda K, Shiraishi T, Yamada T (2000) Independent pathways leading to apoptotic cell death, oxidative burst and defense gene expression in response to elicitin in tobacco. Eur J Biochem 267:5005–4013
Shirasu K, Schulze-Lefert P (2000) Regulator of cell death in disease resistance. Plant Mol Biol 44:371–385
Tada Y, Hata S, Takata Y, Nakayashiki H, Tosa Y, Mayama S (2001) Induction and signaling of an apoptotic response typified by DNA laddering in the defense response of oats to infection and elicitors. Mol Plant Microbe Interact 14:477–486
Tao Y, Xie Z, Chen W, Glazebrook J, Chan HS, Han B, Zhu T, Zou G, Katagiri F (2003) Quantitative nature of Arabidopsis responses during compatible and incompatible interactions with the bacterial pathogen Pseudomonas syringae. Plant Cell 15:317–330
Venisse JS, Gullner G, Brisset MN (2001) Evidence for the involvement of an oxidative stress in the initiation of infection of pear by Erwinia amylovora. Plant Physiol 125:2164–2172
Wang H, Richard MB, Gilchrist DG (1996) Apoptosis: a functional paradigm for programmed plant cell death induced by a host-selective phytotoxin and invoked during development. Plant Cell 8:375–391
Yao N, Imai S, Tada Y, Nakayashiki H, Tosa Y, Park P, Mayama S (2002) Apoptotic cell death in a common response to pathogen attack in oats. Mol Plant Microbe Interact 15:1000–1007
Yao N, Tada Y, Park P, Nakayashiki H, Tosa Y, Mayama S (2001) Novel evidence for apoptotic cell response and differential signals in chromatin condensation and DNA cleavage in victorin-treated oats. Plant J 28:13–26
Acknowledgments
We thank Dr. Yuki Ichinose (Okayama university) and Dr. Kasumi Takeuchi (National Institute of Agriculture) for kindly providing Pseudomonas syringae pv. tabaci 6695. This work was supported by Grants-in Aid for Scientific Research awarded to AK (16780031) and YH (15028214 and 16380037) from the Ministry of Education, Science, Sports and Culture, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kiba, A., Takata, O., Ohnishi, K. et al. Comparative analysis of induction pattern of programmed cell death and defense-related responses during hypersensitive cell death and development of bacterial necrotic leaf spots in eggplant. Planta 224, 981–994 (2006). https://doi.org/10.1007/s00425-006-0277-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-006-0277-1