Abstract
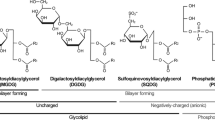
Monogalactosyldiacylglyceride (MGDG) and digalactosyldiacylglyceride (DGDG) are the major membrane lipids of chloroplasts. The question of the specialized functions of these unique lipids has received limited attention. One function is to support violaxanthin de-epoxidase (VDE) activity, an enzyme of the violaxanthin cycle. To understand better the properties of this system, the effects of galactolipids and phosphatidylcholines on VDE activity were examined by two independent methods. The results show that the micelle-forming lipid (MGDG) and bilayer forming lipids (DGDG and phosphatidylcholines) support VDE activity differently. MGDG supported rapid and complete de-epoxidation starting at a threshold lipid concentration (10 μM) coincident with complete solubilization of violaxanthin. In contrast, DGDG supported slow but nevertheless complete to nearly complete de-epoxidation at a lower lipid concentration (6.7 μM) that did not completely solubilize violaxanthin. Phosphotidylcholines showed similar effects as DGDG except that de-epoxidation was incomplete. Since VDE requires solubilized violaxanthin, aggregated violaxanthin in DGDG at low concentration must become solubilized as de-epoxidation proceeds. High lipid concentrations had lower activity possibly due to formation of multilayered structures (liposomes) that restrict accessibility of violaxanthin to VDE. MGDG micelles do not present such restrictions. The results indicate VDE operates throughout the lipid phase of the single bilayer thylakoid membrane and is not limited to putative MGDG micelle domains. Additionally, the results also explain the differential partitioning of violaxanthin between the envelope and thylakoid as due to the relative solubilities of violaxanthin and zeaxanthin in MGDG, DGDG and phospholipids. The violaxanthin cycle is hypothesized to be a linked system of the thylakoid and envelope for signal transduction of light stress.




Similar content being viewed by others
Abbreviations
- A:
-
Antheraxanthin
- Asc:
-
Ascorbate
- DES:
-
De-epoxidation state calculated as A+Z/A+Z+V
- DGDG:
-
Digalactosyldiacylglycerol
- EPC:
-
Egg phosphatidylcholine
- HPLC:
-
High performance liquid chromatography
- MGDG:
-
Monogalactosyldiacylglycerol
- NPQ:
-
Non-photochemical quenching
- PDE:
-
Percent de-epoxidation calculated as (½A+Z/½A+Z+V)×100
- PE:
-
Phosphatidylethanolamine
- SPC:
-
Soy phosphatidylcholine
- V:
-
Violaxanthin
- VDE:
-
Violaxanthin de-epoxidase
- Z:
-
Zeaxanthin
References
Bugos RC, Chang S-W, Yamamoto HY (1999) Developmental expression of violaxanthin de-epoxidase in leaves of tobacco growing under high and low light. Plant Physiol 121:207–213
Demmig B, Winter K, Krüger A, Czygan FC (1987) Photoinhibition and zeaxanthin formation in intact leaves. A possible role of the xanthophyll cycle in the dissipation of excess light energy. Plant Physiol 84:218–224
Demmig-Adams B (2003) Linking the xanthophyll cycle with thermal energy dissipation. Photosyn Res 76:73–80
Demmig-Adams B, Adams III WW, Ebbert V, Logan BA (1999) Ecophysiology of the xanthophyll cycle. In: Frank HA, Young AJ, Britton G, Cogdell RJ (eds) Advances in photosynthesis. The Photochemistry of Carotenoids, vol 8 Kluwer, Dordrecht, pp 245–269
Douce R, Holtz RB, Benson AA (1973) Isolation and properties of the envelope of spinach chloroplasts. J Biol Chem 248:7215–7222
Gilmore AM (2001) Xanthophyll cycle-dependent nonphotochemical quenching in photosystem II: mechanistic insights gained from Arabidopsis thaliana L. mutants that lack violaxanthin deepoxidase activity and/or lutein. Photosyn Res 67:89–101
Gilmore AM,Yamamoto HY (1991) Resolution of lutein and zeaxanthin using a non-endcapped, lightly carbon-loaded C18 high-performance liquid chromatographic column. J Chromatogr 543: 137–145
Goss R, Lohr M, Latowski D, Grzyb J, Vieler A, Wilhelm C, Strzalka K (2005) Role of hexagonal structure-forming lipids in diadinoxanthin and violaxanthin solubilization and de-epoxidation. Biochemistry 44:4028–4036
Havaux M, Niyogi KK (1999) The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc Natl Acad Sci USA 96:8762–8767
Hieber AD, Kawabata O, Yamamoto HY (2004) Significance of the lipid phase in the dynamics and functions of the xanthophyll cycle as revealed by PsbS overexpression in tobacco and in-vitro de-epoxidation in monogalactosyldiacylglycerol micelles. Plant Cell Physiol 45:92–102
Holt NE, Zigmantas D, Valkunas L, Li X-P, Niyogi KK, Fleming GR (2005) Carotenoid cation formation and the regulation of photosynthetic light harvesting. Science 307:433–436
Jin E, Yokthongwattana K, Polle JEW, Melis A (2003) Role of the reversible xanthophyll cycle in the photosystem II damage and repair cycle in Dunaliella salina. Plant Physiol 132:1–13
Külheim C, Agren J, Jansson S (2002) Rapid regulation of light harvesting and plant fitness in the field. Science 297:91–93
Latowski D, Kurk J, Burda K, Skrzynecka-Jaskier M, Kostecka-Gugala A, Strzalka K (2002) Kinetics of violaxanthin de-epoxidation by violaxanthin de-epoxidase, a xanthophyll cycle enzyme, is regulated by membrane fluidity in model lipid bilayers. Eur J Biochem 269:4656–4665
Latowski D, Åkerlund H-E, Strzalka K (2004) Violaxanthin de-epoxidase, the xanthophyll cycle enzyme, requires lipid inverted hexagonal structures for its activity. Biochemistry 43:4417–4420
Mullineaux P, Karpinski S (2002) Signal transduction in response to excess light: getting out of the chloroplast. Curr Opin Plant Biol 5:43–48
Niyogi KK, Li X-P, Rosenberg V, Jung H-S (2004) Is PsbS the site of non-photochemical quenching in photosynthesis? J Exp Bot 56:375–382
Siefermann D, Yamamoto HY (1974) Light-induced de-epoxidation of violaxanthin in lettuce III. Reaction kinetics and effect of light intensity on de-epoxidase activity and substrate availability. Biochim Biophys Acta 357:144–150
Siefermann D, Yamamoto HY (1976) Light-induced de-epoxidation in lettuce chloroplasts VI. De-epoxidation in grana and stroma lamellae. Plant Physiol 57:939–940
Siefermann-Harms D, Joyard J, Douce R (1978) Light-induced changes of the carotenoid levels in chloroplast envelopes. Plant Physiol 61:530–533
Thayer SS, Björkmann O (1990) Leaf xanthophyll content and composition in sun and shade determined by HPLC. Photosyn Res 23:331–343
Sun W-H, Hieber AD, Yamamoto HY (2001a) Overexpression of violaxanthin de-epoxidase inhibits tobacco production. In:Proceedings of the 12th international congress on photosynthesis. CSIRO Publishing S3–038 1–4
Subczynski WK, Markowska E, Gruszecki WI, Sielewiesiuk J (1992) Effects of polar carotenoids on dimyristoylphosphatidylcholine membranes: a spin-label study. Biochim Biophys Acta 1105:97–108
Surpin M, Larkin RM and Chory J (2002) Signal transduction between the chloroplast and the nucleus. Plant Cell S327–S338
Yamamoto HY (1979) Biochemistry of the violaxanthin cycle in higher plants. Pure Appl Chem 51:639–648
Yamamoto HY (1985) Xanthophyll cycles. Methods Enzymol 110:303–312
Yamamoto HY, Bangham AD (1978) Carotenoid organization in membranes. Thermal transition and spectral properties of carotenoid-containing liposomes. Biochim Biophys Acta 507:119–127
Yamamoto HY, Higashi RM (1978) Violaxanthin de-epoxidase. Lipid composition and substrate specificity. Arch Biochim Biophys 190: 514–522
Yamamoto HY, Chenchin EE, Yamada DK (1974) Effect of chloroplast lipids on violaxanthin de-epoxidase activity. In: Avron M (ed) Proceedings of the 3rd international congress on photosynthesis. Elsevier, Amsterdam, pp 1999–2006
Yamamoto HY, Bugos RC, Hieber AD (1999) Biochemistry and molecular biology of the xanthophyll cycle. In: Frank HA, Young AJ, Britton G, Cogdell RJ (eds) Advances in photosynthesis. The photochemistry of carotenoids, vol.8 Kluwer, Dordrecht, pp 293–303
Yokthongwattana K, Savchenko T, Polle JEW, Melis A (2005) Isolation and characterization of a xanthophyll-rich fraction from the thylakoid membrane of Dunaliella salina (green algae). Photochem Photobiol Sci 4:1024–1034
Acknowledgments
This research was supported in part by United States Department of Energy Grant DE-FG03-92ER20078. The author thanks Dr. David Hieber for helpful discussions and Dr. Osamu Kawabata for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamamoto, H.Y. Functional roles of the major chloroplast lipids in the violaxanthin cycle. Planta 224, 719–724 (2006). https://doi.org/10.1007/s00425-006-0257-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-006-0257-5