Abstract
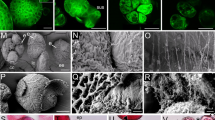
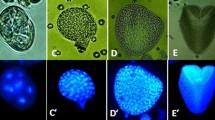
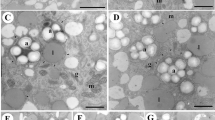
Following abiotic stress to induce barley (Hordeum vulgare L.) androgenesis, the development of 794 enlarged microspores in culture was monitored by time-lapse tracking. In total, 11% of the microspores tracked developed into embryo-like structures (type-I pathway), 36% formed multicellular structures (type-II pathway) and 53% of the microspores followed gametophytic divisions, accumulated starch and died in the first days of tracking (type-III pathway). Despite the microspore fate, enlarged microspores showed similar morphologies directly after stress treatment. Ultrastructural analysis, however, revealed two morphologically distinct cell types. Cells with a thin intine layer and an undifferentiated cytoplasm after stress treatment were associated with type-I and type-II pathways, whereas the presence of differentiated amyloplasts and a thick intine layer were associated with the type-III pathway. Tracking revealed that the first morphological change associated with embryogenic potential was a star-like morphology, which was a transitory stage between uninucleate vacuolated microspores after stress and the initiation of cell division. The difference between type-I and type-II pathways was observed during the time they displayed the star-like morphology. During the transition phase, embryo-like structures in the type-I pathway were always released out of the exine wall at the opposite side of the pollen germ pore, whereas in the type-II pathway multicellular structures were unable to break the exine and to release embryo-like structures. Moreover, by combining viability studies with cell tracking, we show that release of embryo-like structures was preceded by a decrease in viability of the cells positioned at the site of exine wall rupture. These cells were also positively stained by Sytox orange, a cell death indicator. Thereby, we demonstrate, for the first time, that a position-determined cell death process marks the transition from a multicellular structure into an embryo-like structure during barley androgenesis.





Similar content being viewed by others
Abbreviations
- CLSM :
-
Confocal laser scanning microscopy
- ELS :
-
Embryo-like structure released out of the exine wall
- FDA :
-
Fluorescein diacetate
- MCS :
-
Multicellular structure inside the exine wall
- TEM :
-
Transmission electron microscopy
References
Bhalerao RP, Bennett MJ (2003) The case of morphogens in plants. Nature Cell Biol 5:939–943
Bolik M, Koop HU (1991) Identification of embryogenic microspores of barley (Hordeum vulgare L.) by individual selection and culture and their potential for transformation by microinjection. Protoplasma 162:61–68
Bonet FJ, Olmedilla A (2000) Structural changes during early embryogenesis in wheat pollen. Protoplasma 211:94–102
Boutilier KA, Ginés MJ, DeMoor JM, Huang B, Baszczynski CL, Iyer VN, Miki BL (1994) Expression of the BnmNAP subfamily of napin genes coincides with the induction of Brassica microspore embryogenesis. Plant Mol Biol 26:1711–1723
Boutilier K, Offringa R, Sharma VK, Kieft H, Ouellet T, Zhang L, Hattori J, Liu CM, van Lammeren AAM, Miki BLA, Custers JBM, van Lookeren Campagne MM (2002) Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryogenic growth. Plant Cell 14:1737–1749
Bozhkov PV, Filonova LH, Suarez MF, Helmersson A, Smertenko AP, Zhivotovsky, von Arnold S (2004) VEIDase is a principal caspase-like activity involved in plant programmed cell death and essential for embryonic pattern formation. Cell Death Differ 11:175–182
Drews GN, Lee D, Christensen CA (1998) Genetic analysis of female gametophyte development and function. Plant Cell 10:5–17
Filonova LH, Bozhkov PV, von Arnold S (2000a) Developmental pathway of somatic embryogenesis in Picea abies as revealed by time-lapse tracking. J Exp Bot 51:249–264
Filonova LH, Bozhkov PV, Brukhin VB, Daniel G, Zhivotovsky B, von Arnold S (2000b) Two waves of programmed cell death occur during formation and development of somatic embryos in the gymnosperm, Norway spruce. J Cell Sci 113:4399–4411
Gervais G, Newcomb W, Simmonds DH (2000) Rearrangement of the actin filament and microtubule cytoskeleton during induction of microspore embryogenesis in Brassica napus L. cv Topas. Protoplasma 213:194–202
Golds TJ, Babczinsky J, Rauscher G, Koop HU (1992) Computer-controlled tracking of single cell development in Nicotiana tabacum L. and Hordeum vulgare L. protoplasts embedded in agarose/alginate films. J Plant Physiol 140:582–587
Guha S, Maheshwari SC (1964) In vitro production of embryos from anthers of Datura. Nature 204:497
Hoekstra S, van Zijderveld MH, Louwerse JD, Heidekamp F, van der Mark F (1992) Anther and microspore culture of Hordeum vulgare L. cv. Igri. Plant Sci 86:89–96
Hoekstra S, van Zijderveld MH, Heidekamp F, van der Mark F (1993) Microspore culture of Hordeum vulgare L.: the influence of density and osmolarity. Plant Cell Rep 12:661–665
Huang B (1986) Ultrastructural aspects of pollen embryogenesis in Hordeum, Triticum and Paeonia. In: Hu H, Hongyuan Y (eds) Haploids of higher plants in vitro. Springer, Berlin Heidelberg New York, pp 91–117
Indrianto A, Barinova I, Touraev A, Heberle-Bors E (2001) Tracking individual wheat microspores in vitro: identification of embryogenic microspores and body axis formation in the embryo. Planta 212:163–174
Krens FA, Verhoeven HA, van Tunen AJ, Hall RD (1998) The use of an automated cell tracking system to identify specific cell types competent for regeneration and transformation. In vitro Cell Dev Biol Plant 34:81–86
Kumlehn J, Lörz H (1999) Monitoring sporophytic development of individual microspores of barley (Hordeum vulgare L.). In: Clement C, Pacini E, Audran JC (eds) Anther and pollen: from biology to biotechnology. Springer, Berlin Heidelberg New York, pp 183–189
Li H, Devaux P (2001) Enhancement of microspore culture efficiency of recalcitrant barley genotypes. Plant Cell Rep 20:475–481
Magnard JL, Le Deunff E, Domenech J, Rogowsky PM, Testillano PS, Rougier M, Risueño MC, Vergne P, Dumas C (2000) Genes normally expressed in the endosperm are expressed at early stages of microspore embryogenesis in maize. Plant Mol Biol 44:559–574
Maraschin SF, Lamers GEM, de Pater BS, Spaink HP, Wang M (2003) 14-3-3 isoforms and pattern formation during barley microspore embryogenesis. J Exp Bot 51:1033–1043
McCormick S (1993) Male gametophyte development. Plant Cell 5:1265–1275
Mordhorst AP, Toonen MAJ, de Vries SC (1997) Plant embryogenesis. Crit Rev Plant Sci 16:535–576
Paire A, Devaux P, Lafitte C, Dumas C, Matthys-Rochon E (2003) Proteins produced by barley microspores and their derived androgenic structures promote in vitro zygotic maize embryo formation. Plant Cell Tissue Org 73:167–176
Pechan PM, Smykal P (2001) Androgenesis: affecting the fate of the male gametophyte. Physiol Plant 111:1–8
Pennell RI, Lamb C (1997) Programmed cell death in plants. Plant Cell 9:1157–1168
Raghavan V (1986) Pollen embryogenesis. In: Barlow PW, Green PB, Wylie CC (eds) Embryogenesis in angiosperms. Cambridge University Press, Cambridge, pp 153–189
Ramírez C, Testillano PS, Castillo AM, Vallés MP, Coronado MJ, Cistué L, Risueňo MC (2001) The early microspore embryogenesis pathway in barley is accompanied by concrete ultrastructural and expression changes. Int J Dev Biol 45:57–58
Rashid A, Siddiqui AW, Reinert J (1982) Subcellular aspects of origin and structure of pollen embryos of Nicotiana. Protoplasma 113:202–208
Reynolds TL (1997) Pollen embryogenesis. Plant Mol Biol 33:1–10
Simmonds DH, Keller WA (1999) Significance of preprophase bands of microtubules in the induction of microspore embryogenesis of Brassica napus. Planta 208:383–391
Schmidt EDL, Guzzo F, Toonen MAJ, de Vries SC (1997) A leucine-rich repeat containing receptor-like kinase marks somatic plant cells competent to form embryos. Development 124:2049–2062
Smykal PM, Pechan P (2000) Stress, as assessed by the appearance of sHsp transcipts, is required but not sufficient to initiate androgenesis. Physiol Plant 110:135–143
Somleva MN, Schmidt EDL, de Vries SC (2000) Embryogenic cells in Dactylis glomerata L. (Poaceae) explants identified by cell tracking and by SERK expression. Plant Cell Rep 19:718–726
Sterk P, Booij H, Schellekens GA, van Kammen A, de Vries SC (1991) Cell-specific expression of the carrot EP2 lipid transfer protein gene. Plant Cell 3:907–921
Sunderland N, Huang B (1985) Barley anther culture. The switch of programme and albinism. Hereditas 3:27–40
Sunderland N, Roberts M, Evans LJ, Wildon DC (1979) Multicellular pollen formation in cultured barley anthers. I. Independent division of the generative and vegetative cells. J Exp Bot 30:1133–1144
Telmer CA, Newcomb W, Simmonds DH (1995) Cellular changes during heat shock induction and embryo development of cultured microspores of Brassica napus cv. Topas. Protoplasma 185: 106–112
Testillano PS, Ramírez C, Domenech J, Coronado MJ, Vergne P, Matthys–Rochon E, Risueňo MC (2002) Young microspore-derived maize embryos show two domains with defined features also present in zygotic embryogenesis. Int J Dev Biol 46:1035–1047
Toonen MAJ, Hendriks T, Schmidt EDL, Verhoeven HA, van Kammen A, de Vries SC (1994) Description of somatic-embryo-forming single cells in carrot suspension cultures employing video cell tracking. Planta 194:565–572
Touraev A, Vicente O, Heberle-Bors E (1997) Initiation of microspore embryogenesis by stress. Trends Plant Sci 2:297–302
van Bergen S, Kottenhagen MJ, van der Meulen RM, Wang M (1999) Effects of ABA during the pretreatment of barley anthers on androgenesis of Hordeum vulgare L. cultivars Igri and Digger. In: Clement C, Pacini E, Audran JC (eds) Anther and pollen: from biology to biotechnology. Springer, Berlin Heidelberg New York, pp 191–1999
Wang M, van Bergen S, van Duijn B (2000) Insights into a key developmental switch and its importance for efficient plant breeding. Plant Physiol 124:523–530
Yeung EC, Rahman MH, Thorpe TA (1996) Comparative development of zygotic and microspore-derived embryos in Brassica napus L. cv Topas. I. Histodifferentiation. Int J Plant Sci 157:27–39
Wu H, Cheung AY (2000) Programmed cell death in plant reproduction. Plant Mol Biol 44:267–281
Zaki MAM, Dickinson HG (1991) Microspore-derived embryos in Brassica: the significance of division symmetry in pollen mitosis I to embryogenic development. Sex Plant Rep 4:48–55
Acknowledgements
We are grateful to Sandra van Bergen (TNO Department of Applied Plant Sciences, The Netherlands) for technical assistance and valuable discussion and Dr. Wessel de Priester (Institute of Biology Leiden, Leiden University, The Netherlands) for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de F. Maraschin, S., Vennik, M., Lamers, G.E.M. et al. Time-lapse tracking of barley androgenesis reveals position-determined cell death within pro-embryos. Planta 220, 531–540 (2005). https://doi.org/10.1007/s00425-004-1371-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-004-1371-x