Abstract
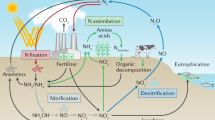
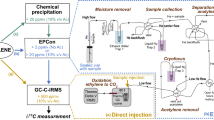
Plants take up inorganic nitrogen and store it unchanged or convert it to organic forms. The nitrogen in such organic compounds is stoichiometrically recoverable by the Kjeldahl method. The sum of inorganic nitrogen and Kjeldahl nitrogen has long been known to equal the total nitrogen in plants. However, in our attempt to study the mechanism of nitrogen dioxide (NO2) metabolism, we unexpectedly discovered that about one-third of the total nitrogen derived from 15N-labeled NO2 taken up by Arabidopsis thaliana (L.) Heynh. plants was converted to neither inorganic nor Kjeldahl nitrogen, but instead to an as yet unknown nitrogen compound(s). We here refer to this nitrogen as unidentified nitrogen (UN). The generality of the formation of UN across species, nitrogen sources and cultivation environments for plants has been shown as follows. Firstly, all of the other 11 plant species studied were found to form the UN in response to fumigation with 15NO2. Secondly, tobacco (Nicotiana tabacum L.) plants fed with 15N-nitrate appeared to form the UN. And lastly, the leaves of naturally fed vegetables, grass and roadside trees were found to possess the UN. In addition, the UN appeared to comprise a substantial proportion of total nitrogen in these plant species. Collectively, all of our present findings imply that there is a novel nitrogen mechanism for the formation of UN in plants. Based on the analyses of the exhaust gas and residue fractions of the Kjeldahl digestion of a plant sample containing the UN, probable candidates for compounds that bear the UN were deduced to be those containing the heat-labile nitrogen–oxygen functions and those recalcitrant to Kjeldahl digestion, including organic nitro and nitroso compounds. We propose UN-bearing compounds may provide a chemical basis for the mechanism of the reactive nitrogen species (RNS), and thus that cross-talk may occur between UN and RNS metabolisms in plants. A mechanism for the formation of UN-bearing compounds, in which RNS are involved as intermediates, is proposed. The important broad impact of this novel nitrogen metabolism, not only on the general physiology of plants, but also on plant substances as human and animal food, and on plants as an integral part of the global environment, is discussed.


Similar content being viewed by others
Abbreviations
- NO :
-
Nitric oxide
- NO 2 :
-
Nitrogen dioxide
- RNS :
-
Reactive nitrogen species
- UN :
-
Unidentified nitrogen
- TNNAT, RNNAT, INNAT and UNNAT :
-
Total, Kjeldahl, inorganic and unidentified nitrogen in naturally fed plants, respectively
- TNNIT, RNNIT, INNIT and UNNIT :
-
Total, Kjeldahl, inorganic and unidentified nitrogen derived from nitrate, respectively
- TNNO2, RNNO2, INNO2 and UNNO2 :
-
Total, Kjeldahl, inorganic and unidentified nitrogen derived from NO2, respectively
References
Amman M, Stalder M, Suter M, Brunold C, Baltensperger U, Jost T, Turler A, Gaggeler W (1995) Tracing uptake and assimilation of NO2 in spruce needles with 13N. J Exp Bot 46:1685–1691
Arimoto J, Morikawa H, Takahashi M, Shigematsu Y, Fujita K (2003) Plant nutritional and physiological study on the unidentified nitrogen (UN) in gramineous and leguminous grass. Abstracts of the annual meeting, Japanese society of soil science and plant nutrition, 20–22 August 2003, Kanagawa, Japan, vol 49, p 95
Beckman JS (1996) Nitric oxide. In: Lancaster J Jr (ed) Principles and actions. Academic Press, San Diego, pp 1–82
Beligni MV, Lamattina L (2001) Nitric oxide in plants: the history is just beginning. Plant Cell 24:267–278
Bourgin JP, Chupeau Y, Missonier C (1979) Plant regeneration from mesophyll protoplasts of several Nicotiana species. Physiol Plant 45:288–292
Bradstreet RB (1965) The Kjeldahl digestion. In: Bradstreet RB (ed) The Kjeldahl method for organic nitrogen. Academic Press, New York, pp 9–88
Bruning-Fann CS, Kaneene JB (1993) The effects of nitrate, nitrite and N-nitroso compounds on animal health: a review. Vet Hum Toxicol 35:521–538
Brunori M, Giuffre A, Sarti P, Stubauer G, Wilson MT (1999) Nitric oxide and cellular respiration. Cell Mol Life Sci 56:549–557
Chandok MR, Ytterberg AJ, van Wijk KJ, Klessig DF (2003) The pathogen-inducible nitric oxide synthetase (iNOS) in plants is a variant of the P protein of the glycine decarboxylase complex. Cell 113:469–482
Chung HT, Pae HO, Choi BM, Billiar TR, Kim YM (2001) Nitric oxide as a bioregulator of apoptosis. Biochem Biophys Res Commun 282:1075–1079
Conway EJ, Byrne A (1933) An absorption apparatus for the micro-determination of certain volatile substances. I. The micro-determination of ammonia. Biochem J 27:419–429
Delledonne M, Xia Y, Dixon RA, Lamb C (1998) Nitric oxide functions as a signal in plant disease resistance. Nature 394:585–588
Durner J, Klessig DF (1999) Nitric oxide as a signal in plants. Curr Opin Plant Biol 2:369–374
Fan AM, Steinberg VE (1996) Health implications of nitrate and nitrite in drinking water: an update on methemoglobinemia occurrence and reproductive and developmental toxicity. Regul Toxicol Pharmacol 23:35–43
Gatley SJ, Shea C (1991) Radiochemical and chemical quality-assurance methods for [13N]-ammonia made from a small volume H2 16O target. Appl Radiat Isot 42:793–796
Goldstein S, Czapski G, Lind J, Merenyi G (2000) Tyrosine nitration by simultaneous generation of NO and O2 under physiological conditions. J Biol Chem 275:3031–3036
Goshima N, Mukai T, Suemori M, Takahashi M, Caboche M, Morikawa H (1999) Emission of nitrous oxide (N2O) from transgenic tobacco expressing antisense nitrite reductase mRNA. Plant J 19:75–80
Hill MJ (1999) Nitrate toxicity: myth or reality? Br J Nutr. 81:343–344
Kawamura Y, Takahashi M, Ariumura G, Isayama T, Irifune K, Goshima N, Morikawa H (1996) Determination of levels of NO3 −, NO2 −, and NH4+ ions in leaves of various plants by capillary electrophoresis. Plant Cell Physiol 37:878–880
Larcher W (1995) Plants under stress. In: Larcher W (ed) Physiological plant ecology, 3rd edn. Springer, Berlin Heidelberg New York, pp 321–448
Lea PJ (1993) Nitrogen metabolism. In: Lea PJ, Leegood RC (eds) Plant biochemistry and molecular biology, 2nd edn. Wiley, Chichester, pp 163–191
Liu L, Hausladen A, Zeng M, Que L, Heitman J, Stamler JS (2001) A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature 410:490–494
Mariotti A (1983) Atmospheric nitrogen is a reliable standard for natural 15N abundance measurements. Nature 303:685–687
Marzinzig M, Nussler AK, Stadler J, Marzinzig E, Barthlen W, Nussler NC, Beger HG, Morris SM Jr, Bruckner UB (1997) Improved methods to measure end products of nitric oxide in biological fluids: nitrite, nitrate, and S-nitrosothiols. Nitric Oxide Biol Chem 1:177–189
Mata CG, Lamattina L (2002) Nitric oxide and abscisic acid cross talk in guard cells. Plant Physiol 128:790–792
McDowell JJ, Dangl JL (2000) Signal transduction in the plant immune response. Trends Biochem Sci 25:79–82
McKnight GM, Duncan CW, Leifert C, Golden MH (1999) Dietary nitrate in man: friend or foe? Br J Nutr 81:349–358
Morikawa H, Higaki A, Nohno M, Takahashi M, Kamada M, Nakata M, Toyohara G, Okaumra Y, Matsui K, Kitani S, Fujita K, Irifune K, Goshima N (1998a) More than a 600-fold variation in nitrogen dioxide assimilation among 217 plant taxa. Plant Cell Environ 21:180–190
Morikawa H, Takahashi M, Irifune K (1998b) Molecular mechanism of the metabolism of nitrogen dioxide as and an alternative fertilizer in plants. In: Satoh K, Murata N (eds) Stress responses of photosynthetic organisms. Elsevier, Amsterdam, pp 227–237
Mulvaney CS, Hageman RH (1984) Acetaldehyde oxime, a product formed during the in vivo nitrate reductase assay of soybean leaves. Plant Physiol 76:118–124
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nathan C, Shiloh MU (2000) Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc Natl Acad Sci USA 97:8841–8848
Neill SJ, Desikan R, Clarke A, Hancock JT (2002) Nitric oxide is a novel component of abscisic acid signaling in stomatal guard cells. Plant Physiol 128:13–16
Nonoyama N, Chiba K, Hisatome K, Suzuki H, Shintani F (1999) Nitration and hydroxylation of substituted phenols by peroxynitrite. Kinetic feature and an alternative mechanistic view. Tetrahedron Lett 40:6933–6937
Rockel P, Strube F, Rockel A, Wildt J, Kaiser WM (2002) Regulation of nitric oxide (NO) production by plant nitrate reductase in vivo and in vitro. J Exp Bot 53:103–110
Sakamoto A, Ueda M, Morikawa H (2002) Arabidopsis glutathione-dependent formaldehyde dehydrogenase is an S-nitrosoglutathione reductase. FEBS Lett 515:20–24
Sakamoto A, Tsukamoto S, Yamamoto H, Ueda–Hashimoto M, Takahashi M, Suzuki H, Morikawa H (2003) Functional complementation in yeast reveals a protective role of chloroplast 2-Cys peroxiredoxin against reactive nitrogen species. Plant J 33:841–851
Sanchez-Echaniz J, Benito-Fernandez J, Mintegui-Raso S (2001) Methemoglobinemia and consumption of vegetables in infants. Pediatrics 107:1024–1028
Sawa T, Akaike T, Maeda H (2000) Tyrosine nitration by peroxynitrite formed from nitric oxide and superoxide generated by xanthine oxidase. J Biol Chem 275:32467–32474
Shigematsu Y, Morikawa H, Takahashi M, Arimoto J, Fujita K (2003) Plant nutritional and physiological study on the unidentified nitrogen (UN) in vegetable crops. Abstracts of the annual meeting, Japanese society of soil science and plant nutrition, 20–22 August 2003, Kanagawa, Japan, vol 49, p 95
Stamler JS (1994) Redox signaling: nitrosylation and related target interactions of nitric oxide. Cell 78:931–936
Stamler JS, Lamas S, Fang FC (2001) Nitrosylation: the prototypic redox-based signaling mechanism. Cell 106:675–683
Takahashi M, Sasaki Y, Ida S, Morikawa H (2001) Enrichment of nitrite reductase gene improves the ability of Arabidopsis thaliana plants to assimilate nitrogen dioxide. Plant Physiol 126:731–741
Takahashi M, Kondo K, Morikawa H (2003) Assimilation of nitrogen dioxide in selected plant taxa. Acta Biotechnol 23:241–247
Tun NN, Holk A, Scherer GFE (2001) Rapid increase of NO release in plant cell cultures induced by cytokinin. FEBS Lett 509:174–176
Van Camp W, Van Montagu M, Inze D (1998) H2O2 and NO: redox signals in disease resistance. Trends Plant Sci 3:330–334
Vaucheret H, Kronenberger J, Lepingle A, Vilaine F, Boutin JP, Caboche M (1992) Inhibition of tobacco nitrite reductase activity by expression of antisense RNA. Plant J 2:559–569
Wendehenne D, Pugin A, Klessig DF, Durner J (2001) Nitric oxide: comparative synthesis and signaling in animal and plant cells. Trends Plant Sci 6:177–183
Yamasaki H, Sakihama Y, Takahashi S (1999) An alternative pathway for nitric oxide production in plants: new features of an old enzyme. Trends Plant Sci 4:128–129
Acknowledgements
We thank T. Sugiyama of Nagoya University and T. Yamaya of Tohoku University for discussions on nitrogen metabolism, and M. Kawahara of Hiroshima University for nitrogen analysis. This work was supported in part by the Research for the Future Program, Japanese Society for the Promotion of Science (JSPS-RFTF96L00604) and by a Grant-in-Aid for Creative Scientific Research (no.13GS0023) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morikawa, H., Takahashi, M., Sakamoto, A. et al. Formation of unidentified nitrogen in plants: an implication for a novel nitrogen metabolism. Planta 219, 14–22 (2004). https://doi.org/10.1007/s00425-003-1200-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-003-1200-7