Abstract
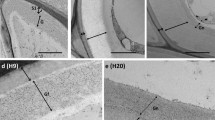
Polygalacturonase (PG) is a cell wall-associated protein that degrades pectin. A ZePG1 cDNA encoding a putative PG was isolated from Zinnia elegens L. and a rabbit antibody specific to the ZePG1 protein was generated. The level of the ZePG1 protein was up-regulated when tracheary element differentiation was initiated. Using gold-labeled secondary antibodies for light and electron microscopy, ZePG1 protein was localized in cultured Zinnia cells. This protein was preferentially distributed on tracheary elements (TEs). At the subcellular level, the protein was localized on secondary wall thickenings, primary walls, Golgi bodies and vesicles. Thus, the putative role of the ZePG1 protein might be the degradation of pectic substances before lignification. Some non-TE cells also accumulated ZePG1 protein on primary walls, Golgi bodies and vesicles. The accumulation of ZePG1 protein on primary walls seems to be at the elongating tips of non-TE cells. In plants, ZePG1 protein was localized on the secondary wall thickenings of differentiating TEs and phloem regions. These results suggest that the expression of the ZePG1 protein is highly regulated both spatially and temporally during in vitro and in situ TE differentiation.









Similar content being viewed by others
Abbreviations
- GST :
-
Glutathione-S-transferase
- PATAg :
-
Periodic acid–thiocarbohydrazide–silver proteinate
- PG :
-
Polygalacturonase
- TE :
-
Tracheary element
References
Allen RL, Lonsdale DM (1992) Sequence analysis of three members of the maize polygalacturonase gene family expressed during pollen development. Plant Mol Biol 20:343–345
Bonghi C, Rascio N, Ramina A, Casadoro G (1992) Cellulase and polygalacturonase involvement in the abscission of leaf and fruit explants of peach. Plant Mol Biol 20:839–848
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of dye binding. Anal Biochem 72:248–254
Brown SM, Crouch ML (1990) Characterization of a gene family abundantly expressed in Oenothera organensis pollen that shows sequence similarity to polygalacturonase. Plant Cell 2:263–274
Burgess J, Linstead P (1984) In-vitro tracheary element formation. Structural studies and the effect of tri-iodobenzoic acid. Planta 160:481–489
Carpita NC, Gibeaut DM (1993) Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the cell walls during growth. Plant J 3:1–30
Demura T, Tashiro G, Horiguchi G, Kishimoto N, Kubo M, Matsuoka N, Minami A, Nagata-Hiwatashi M, Nakamura K, Okamura Y, Sassa N, Suzuki S, Yazaki J, Kikuchi S, Fukuda H (2002) Visualization by comprehensive microarray analysis of gene expression programs during transdifferentiation of mesophyll cells into xylem cells. Proc Natl Acad Sci USA 99:15794–15799
Dubald M, Barakate A, Mandaron P, Mache R (1993) The ubiquitous presence of exopolygalacturonase in maize suggests a fundamental cellular function for this enzyme. Plant J 4:781–791
Esau K, Charvat I (1978) On vessel member differentiation in the (kidney) bean (Phaseolus vulgaris L.). Ann Bot 42:665–677
Fischer RL, Bennett AB (1991) Role of cell wall hydrolases in fruit ripening. Annu Rev Plant Physiol 42:675–703
Fry SC (1995) Polysaccharide-modifying enzymes in the plant cell wall. Annu Rev Plant Physiol Plant Mol Biol 46:497–520
Fukuda H (1997) Tracheary element differentiation. Plant Cell 9:1147–1156
Fukuda H, Komamine A (1980) Establishment of an experimental system for the study of tracheary element differentiation from single cells isolated from the mesophyll of Zinnia elegans. Plant Physiol 65:57–60
Hadfield KA, Bennett AB (1998) Polygalacturonases: many genes in search of a function. Plant Physiol 117:337–343
Hawes MC, Lin H (1990) Correlation of pectolytic enzyme activity with the programmed release of cells from root caps of pea (Pisum sativum). Plant Physiol 94:1855–1859
Hsu SU, Raine L, Fanger H (1981) Use of avidin–biotin–peroxidase complex (ABC) in immunoperoxidase techniques. A comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 29:577–580
Kalaitzis P, Solomos T, Tucker ML (1997) Three different polygalacturonases are expressed in tomato leaf and flower abscission, each with a different temporal expression pattern. Plant Physiol 113:1021–1028
Konno H, Tsumuki H (1991) An exo-polygalacturonase from rice shoots. Phytochemistry 30:2115–2118
Konno H, Yamasaki Y, Katoh K (1989) Extracellular exo-polygalacturonase secreted from carrot cell cultures. Its purification and involvement in pectic polymer degradation. Physiol Plant 76:514–520
Murmanis LL (1978) Breakdown of end wall in differentiating vessels of secondary xylem in Quercus rubra L. Ann Bot 42:679–682
Nakashima J, Awano T, Takabe K, Fujita M, Saiki H (1997) Immunocytochemical localization of phenylalanine ammonia-lyase and cinnamyl alcohol dehydrogenase in differentiating tracheary elements derived from Zinnia mesophyll cells. Plant Cell Physiol 38:113–123
Niogret MF, Dubald M, Mandaron P, Mache R (1991) Characterization of pollen polygalacturonase encoded by several cDNA clones in maize. Plant Mol Biol 17:1155–1164
Ohdaira Y, Kakegawa K, Amino S, Sugiyama M, Fukuda H (2002) Activity of cell-wall autolysis associated with differentiation of isolated mesophyll cells of Zinnia elegans into tracheary elements. Planta 215:177–184
Pressey R, Avants JK (1973) Two forms of polygalacturonase in tomato. Biochim Biophys Acta 309:363–369
Pressey R, Avants JK (1977) Occurrence and properties of polygalacturonase in Avena and other plants. Plant Physiol 60:548–553
Schagger H, Von Jagow G (1987) Tricine–SDS polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 166:368–379
Sitrit Y, Downie B, Bennett AB, Bradford KJ (1996) A novel exo-polygalacturonase is associated with radicle protrusion in tomato (Lycopersicon esculentum) seeds (abstract No. 752). Plant Physiol [Suppl] 111:161
Sugiyama M, Fukuda H, Komamine A (1986) Effects of nutrient limitation and gamma-irradiation on tracheary element differentiation and cell division in single mesophyll cells of Zinnia elegans. Plant Cell Physiol 27:601–606
Taylor JE, Webb STJ, Coupe SA, Tucker GA, Roberts JA (1993) Changes in polygalacturonase activity and solubility of polyuronides during ethylene-stimulated leaf abscission in Sambucus nigra. J Exp Bot 44:93–98
Tebbutt SJ, Rogers HJ, Lonsdale DM (1994) Characterization of a tobacco gene encoding a pollen-specific polygalacturonase. Plant Mol Biol 25:283–297
Thiery JP (1967) Mise en evidence des polysaccharides sur coupes fines en microscopie electronique. J Microsc 6:987–1018
Tucker GA, Schindler BC, Roberts JA (1984) Flower abscission in mutant tomato plants. Planta 160:164–167
Acknowledgements
The authors express their sincere thanks to Dr. Dwight K. Romanovicz for kindly reading this manuscript and providing valuable suggestions. The authors express their special thanks to Prof. Roland M. Brown Jr. for reading this manuscript and providing helpful comments. The authors also thank Dr. Munetaka Sugiyama for supplying ZePG1 cDNA and sharing his unpublished data. This work was supported in part by Grants-in-Aid from the Ministry of Education, Science, Sports, and Culture of Japan (Nos. 10219201 and 14036205 to H.F.), and from the Japan Society for the Promotion of Science (13440236 to H.F.), from the Mitsubishi Foundation to H.F., and by the Japan Society for the Promotion of Science Research Fellowships for Young Scientists (to N.S.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakashima, J., Endo, S. & Fukuda, H. Immunocytochemical localization of polygalacturonase during tracheary element differentiation in Zinnia elegans . Planta 218, 729–739 (2004). https://doi.org/10.1007/s00425-003-1167-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-003-1167-4