Abstract
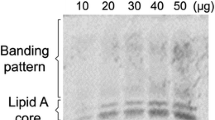
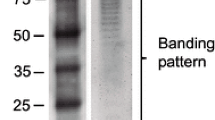
Lipopolysaccharides (LPS) are cell surface components of Gram-negative bacteria and, as microbe- / pathogen-associated molecular patterns, have diverse roles in plant–microbe interactions, e.g. LPS are able to promote plant disease tolerance through activation of induced or acquired resistance. However, little is known about the mechanisms of signal perception and transduction in response to elicitation by these bio-active lipoglycans. The present study focused on the involvement of LPS isolated from the outer cell wall of the Gram-negative bacterium Burkholderia cepacia (strain ASP B 2D) in the molecular mechanisms and components involved in signal perception and transduction and defense-associated responses in suspension-cultured tobacco (Nicotiana tabacum L.) cells. The purified LPSB.cep. was found to trigger a rapid influx of Ca2+ into the cytoplasm of aequorin-transformed tobacco cells. An oxidative burst, concomitant with the production of reactive oxygen and nitrogen species was measured by chemiluminescence and fluorescence. These early perception responses were accompanied by K+/H+ exchange and alkalinization of the extracellular medium. Through the use of various inhibitors of the oxidative burst reaction, as well as scavengers of produced radicals, the biochemical basis of the cellular response to LPSB.cep. elicitation was dissected, elucidated and compared to that induced by a yeast elicitor. These results suggest that LPSB.cep. interacts with tobacco cells in a manner different from the response elicited by yeast elicitor.






Similar content being viewed by others
Abbreviations
- DDC :
-
Diethyldithiocarbamate
- DMSO :
-
Dimethyl sulfoxide
- DPI :
-
Diphenylene iodonium
- H 2 DCF-DA :
-
2′,7′-Dihydrodichlorofluorescein-diacetate
- LPS :
-
Lipopolysaccharides
- NAC :
-
N-Acetyl-l-cysteine
- PTIO :
-
2-Phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide
- ROS :
-
Reactive oxygen species
- YE :
-
Yeast elicitor
References
Albus U, Baier R, Holst O, Pühler A, Niehaus K (2001) Suppression of an elicitor-induced oxidative burst reaction in Medicago sativa cell cultures by Sinorhizobium meliloti lipopolysaccharides. New Phytol 151:597–606
Alexander C, Rietschel ET (2001) Bacterial lipopolysaccharides and innate immunity. J Endotoxin Res 7:167–202
Allan AC, Fluhr R (1997) Two distinct sources of elicited reactive oxygen species in tobacco epidermal cells. Plant Cell 9:1559–1572
Beveridge TJ (1999) Structures of Gram-negative cell walls and their derived membrane vesicles. J Bacteriol 181:4725–4733
Cao H, Baldini RL, Rahme LG (2001) Common mechanisms for pathogens of plants and animals. Annu Rev Phytopathol 39:259–284
Cessna SG, Low PS (2001) Activation of the oxidative burst in aequorin-transformed Nicotiana tabacum cells is mediated by protein kinase- and anion channel-dependent release of Ca2+ from internal stores. Planta 214:126–134
Chandra S, Low PS (1997) Measurement of Ca2+ fluxes during elicitation of the oxidative burst in aequorin-transformed tobacco cells. J Biol Chem 272:28274–28280
Coventry HS, Dubery IA (2001) Lipopolysaccharides from Burkholderia cepacia contribute to an enhanced defense capacity and the induction of pathogenesis-related proteins in Nicotiana tabacum. Physiol Mol Plant Pathol 58:149–158
Delledonne M, Zeier J, Marocco A, Lamb C (2001) Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc Natl Acad Sci USA 98:13454–13459
Dow M, Newman M-A, von Roepenack E (2000) The induction and modulation of plant defense responses by bacterial lipopolysaccharides. Annu Rev Phytopathol 38:241–261
Felix G, Regenass M, Boller T (1993) Specific perception of subnanomolar concentrations of chitin fragments by tomato cells: induction of extracellular alkalinization, changes in protein phosphorylation, and establishment of a refractory state. Plant J 4:307–316
Felix G, Duran JD, Volko S, Boller T (1999) Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J 18:265–276
Foissner I, Wendehenne D, Langebartels C, Durner J (2000) In vivo imaging of an elicitor-induced nitric oxide burst in tobacco. Plant J 23:817–824
Gerber IB, Dubery IA (2002) Enhanced detection of chemiluminescence intensity during an oxidative burst reaction in suspension cultured Nicotiana tabacum cells through the use of Ficoll. Anal Biochem 306:152–154
Gerber IB, Dubery IA (2003) Fluorescence microplate assay for the detection of oxidative burst products in tobacco cell suspensions using 2′,7′-dichlorofluorescein. Methods Cell Sci (in press)
Heikkila RE, Cabbat FS, Cohen G (1976) In vivo inhibition of superoxide dismutase in mice by diethyldithiocarbamate. J Biol Chem 251:2182–2185
Hempel SL, Buettner GR, O’Malley YQ, Wessels DA, Flaherty DM (1999) Dihydro-fluorescein diacetate is superior for detecting intracellular oxidants: comparison with 2′,7′-dichlorodihydrofluorescein diacetate, 5 (and 6)-carboxy-2′,7′-dichloro-dihydrofluorescein diacetate, and dihydrorhodamine 123. Free Rad Biol Med 27:146–159
Keston AS, Brandt R (1965) Fluorometric analysis of ultramicro quantities of hydrogen peroxide. Anal Biochem 11:1–5
Knight MR, Campbell AK, Smith SM, Trewavas AJ (1991) Transgenic plant aequorin reports the effects of touch and cold shock and elicitors on cytoplasmic calcium. Nature 352:524–526
McDowell JM, Dangl JL (2000) Signal transduction in the plant immune response. Trends Biochem Sci 25:79–82
Meyer A, Pühler A, Niehaus K (2001) The lipopolysaccharides of the phytopathogen Xanthomonas campestris pv. campestris induce an oxidative burst reaction in cell cultures of Nicotiana tabacum. Planta 213:214–222
Mithöfer A, Ebel J, Baghwat AA, Neuhaus-Url G (1999) Transgenic aequorin monitors cytosolic calcium transients in soybean challenged with β-glucan or chitin elicitors. Planta 207:566–574
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Newman MA, von Roepenack E, Daniels MJ, Dow JM (2000) Lipopolysaccharides and plant responses to phytopathogenic bacteria. Mol Plant Pathol 1:25–31
Newman MA, von Roepenack-Lahaye E, Parr A, Daniels MJ, Dow M (2002) Prior exposure to lipopolysaccharides potentiates expression of plant defenses in response to bacteria. Plant J 29:487–495
Nürnberger T, Brunner F (2002) Innate immunity in plants and animals: emerging parallels between the recognition of general elicitors and pathogen-associated molecular patterns. Curr Opin Plant Biol 5:1–7
O’Donnel VB, Tew DG, Jones OTG, England PJ (1993) Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem J 290:41–49
Otte O, Pachten A, Hein F, Barz W (2001) Early elicitor-induced events in chickpea cells: functional links between oxidative burst, sequential occurrence of extracellular alkalinization and acidification, K+/H+ exchange and defense-related gene activation. Z Naturforsch Teil C 56:65–76
Pachten A, Barz W (1999) Elicitor-stimulated oxidative burst and extracellular pH changes in protoplast suspensions prepared from cultured chickpea (Cicer arietinum L) cells. J Plant Physiol 155:795–797
Pfeiffer S, Leopold E, Hemmins B, Schmidt K, Werner ER, Mayer B (1997) Interference of carboxy-PTIO with nitric oxide- and peroxynitrite-mediated reactions. Free Rad Biol Med 22:787–794
Possel H, Noack H, Augustin W, Keilhoff G, Wolf G (1997) 2,7-Dihydrodichloro-fluorescein diacetate as a fluorescent marker for peroxynitrite formation. FEBS Lett 416:175–178
Rosenkranz AR, Schmaldienst S, Stuhlmeier KM, Chen W, Knapp W, Zlabinger GJ (1992) A microplate assay for the detection of oxidative products using 2′,7′-dichlorofluorescein-diacetate. J Immunol Methods 156:39–45
Roswell DF, White EH (1978) The chemiluminescence of luminol and related hydrazides. Methods Enzymol 57:409–483
Scheel D (1998) Resistance response physiology and signal transduction. Curr Opin Plant Biol 1:305–310
Schromm AB, Brandenburg K, Loppnow H, Moran AP, Koch MHJ, Rietschel ET, Seydel U (2000) Biological activities of lipopolysaccharides are determined by the shape of their lipid A portion. Eur J Biochem 267:2008–2013
Somssich IK, Hahlbrock K (1998) Pathogen defense in plants—a paradigm of biological complexity. Trends Plant Sci 3:86–90
Trewavas A (2000) Signal perception and transduction. In: Buchanan B, Gruissem W, Jones R (eds) Biochemistry and molecular biology of plants. American Society of Plant Physiologists, Rockville, USA, pp 930–986
Trewavas AJ, Knight M (1994) Mechanical signaling, calcium and plant form. Plant Mol Biol 26:1329–1341
Triantafilou M, Triantafilou K (2002) Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends Immunol 23:301–304
Van Loon LC, Bakker PAHM, Pieterse CMJ (1998) Systemic resistance induced by rhizosphere bacteria. Annu Rev Phytopathol 36:453–483
Westphal O, Jann K (1965) Bacterial lipopolysaccharides: Extraction with phenol-water and further applications of the procedure. Methods Carbohydr Chem 5:83–91
Acknowledgements
We thank Dr. T. Nürnberger (Institute for Plant Biochemistry, Halle, Germany) for valuable advice and cooperation and Dr. J. Jones (John Innes Research Institute, Norwich, UK) for the aequorin-transformed cells. This research was supported by grants from the Deutsche Forschungsgemeinschaft, DFG, SPP 1110, Innate Immunity (J.D.), the South African National Research Foundation, NRF, (I.A.D.) and the Volkswagen Foundation (I.A.D.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gerber, I.B., Zeidler, D., Durner, J. et al. Early perception responses of Nicotiana tabacum cells in response to lipopolysaccharides from Burkholderia cepacia . Planta 218, 647–657 (2004). https://doi.org/10.1007/s00425-003-1142-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-003-1142-0