Abstract
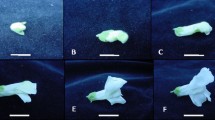
Allene oxide synthase (AOS) catalyzes the entrance reaction in the biosynthesis of the octadecanoids 12-oxophytodienoic acid (OPDA) and jasmonic acid (JA). The enzyme is feedback-regulated by JA and thus a target of the JA-signalling pathway. A fusion genetic approach was used to isolate mutants in this signalling pathway. Seeds from transgenic Arabidopsis thaliana plants expressing the Escherichia coli uidA gene encoding β-glucuronidase (GUS) under the control of the AOS promoter were mutagenized with ethylmethane sulfonate and the progeny was screened for individuals exhibiting constitutive expression of uidA in the absence of an added octadecanoid. From 21,000 mutagenized plants, 8 lines showing constitutive AOS expression were obtained. The mutant lines were characterized further and fell into four classes, I to IV. All showed signs of growth inhibition encompassing both shoot and root systems, and accumulated higher than normal levels of OPDA. Mutants belonging to classes I and IV failed to set seeds due to defects in flower development which prevented self-pollination. One mutant, designated cas1, was characterized in more detail and showed, in addition to elevated levels of AOS mRNA, AOS polypeptide, OPDA, and JA, constitutive expression of JA-responsive genes (VSP2, PDF1.2). The cas1 mutation is recessive and affects a single locus. Using cleaved amplified polymorphic sequences (CAPS) and simple sequence length polymorphisms (SSLP), the mutated gene was mapped to chromosome IV next to the SSLP marker CIW7.




Similar content being viewed by others
Abbreviations
- AOS:
-
allene oxide synthase
- CAPS:
-
cleaved amplified polymorphic sequences
- CAS:
-
constitutive allene oxide synthase
- GUS:
-
β-glucuronidase
- JA:
-
jasmonic acid
- MeJA:
-
methyl jasmonate
- MUG:
-
methylumbelliferyl-β-d-glucuronide
- OPDA:
-
12-oxophytodienoic acid
- SSLP:
-
simple sequence length polymorphisms
References
Barkan A (1989) Tissue dependent plastid RNA splicing in maize: transcripts from four plastid genes are predominantly unspliced in leaf meristems and roots. Plant Cell 1:437–445
Bell CJ, Ecker JR (1994) Assignment of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics 19:137–144
Bell E, Mullet JE (1993) Characterization of an Arabidopsis lipoxygenase gene responsive to methyl jasmonate and wounding. Plant Physiol 103:1133–1137
Bell E, Creelman R, Mullet JE (1995) A chloroplast lipoxygenase is required for wound-induced jasmonic acid accumulation in Arabidopsis. Proc Natl Acad Sci USA 92:8675–8679
Berger S (2002) Jasmonate-related mutants of Arabidopsis as tools for studying stress signaling. Planta 214:497–504
Berger S, Bell E, Mullet JE (1996) Two methyl jasmonate-insensitive mutants show altered expression of AtVsp in response to methyl jasmonate and wounding. Plant Physiol 111:525–531
Blechert S, Bockelmann C, Füsslein M, von Schrader T, Stelmach B, Niesel U, Weiler EW (1999) Structure–activity analyses reveal the existence of two separate groups of octadecanoids in elicitation of the tendril-coiling response of Bryonia dioica Jacq. Planta 207:470–479
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Ellis C, Turner JG (2001) The Arabidopsis mutant cev1 has constitutively active jasmonate and ethylene signal pathways and enhanced resistance to pathogens. Plant Cell 13:1025–1033
Farmer EE, Ryan CA (1990) Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc Natl Acad Sci USA 87:7713–7716
Feys BJF, Benedetti CE, Penfold CN, Turner JG (1994) Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell 6:751–759
Gong Z, Koiwa H, Cushman MA, Ray A, Bufford D, Kore-Eda S, Matsumoto TK, Zhu J, Cushman J, Bressan RA, Hasegawa PM (2001) Genes that are uniquely stress regulated in salt overly sensitive (sos) mutants. Plant Physiol 126:363–375
Gundlach H, Müller MJ, Kutchan TM, Zenk MH (1992) Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proc Natl Acad Sci USA 89:2389–2393
Harms K, Atzorn R, Brash A, Kühn H, Wasternack C, Willmitzer L, Pena-Cortés H (1995) Expression of a flax allene oxide synthase cDNA leads to increased endogenous jasmonic acid (JA) levels in transgenic potato plants but not to a corresponding activation of JA-responding genes. Plant Cell 7:1645–1654
Hilpert B, Bohlmann H, op den Camp R, Przybyla D, Miersch O, Buchala A, Apel K (2001) Isolation and characterization of signal transduction mutants of Arabidopsis thaliana that constitutively activate the octadecanoid pathway and form necrotic microlesions. Plant J 26:435–446
Ishiguro S, Kawai-Okada A, Ueda J, Nishida I, Okada K (2001) The DEFECTIVE IN ANTHER DEHISCENCE1 gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. Plant Cell 13:2191–2209
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Jensen AB, Raventos, D Mundy J (2002) Fusion genetic analysis of jasmonate-signalling mutants in Arabidopsis. Plant J 29:595–606
Konieczny A, Ausubel FM (1993) A procedure for mapping Arabidopsis mutations using co-dominant ecotype-specific PCR-based markers. Plant J 4:403–410
Kubigsteltig I, Laudert D, Weiler EW (1999) Structure and regulation of the Arabidopsis thaliana allene oxide synthase gene. Planta 208:463–471
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Laudert D, Weiler EW (1998) Allene oxide synthase: a major control point in Arabidopsis thaliana octadecanoid signalling. Plant J 15:675–684
Laudert D, Pfannschmidt U, Lottspeich F, Holländer-Czytko H, Weiler EW (1996) Cloning, molecular and functional characterization of Arabidopsis thaliana allene oxide synthase (CYP 74), the first enzyme of the octadecanoid pathway to jasmonates. Plant Mol Biol 31:323–335
Laudert D, Schaller F, Weiler EW (2000) Transgenic Nicotiana tabacum and Arabidopsis thaliana plants overexpressing allene oxide synthase. Planta 211:163–165
McConn M, Browse J (1996) The critical requirement for linolenic acid is pollen development, not photosynthesis, in an Arabidopsis mutant. Plant Cell 8:403–416
McConn M, Creelman RA, Bell E, Mullet JE, Browse J (1997) Jasmonate is essential for insect defense in Arabidopsis. Proc Natl Acad Sci USA 94:5473–5477
Müller A, Düchting P, Weiler EW (2002) A multiplex GC–MS/MS technique for the sensitive and quantitative single-run analysis of acidic phytohormones and related compounds, and its application to Arabidopsis thaliana. Planta 216:44–56
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Murray MG, Thompson WF (1980) Rapid isolation of high-molecular-weight plant DNA. Nucleic Acids Res 8:4321–4325
Park J-H, Halitschke R, Kim HB, Baldwin IT, Feldmann KA, Feyereisen R (2002) A knock-out mutation in allene oxide synthase results in male sterility and defective wound signal transduction in Arabidopsis due to a block in jasmonic acid biosynthesis. Plant J 31:1—12
Penninckx IA, Eggermont K, Terras FR, Thomma BP, De Samblanx GW, Buchala A, Metraux JP, Manners JM, Broekaert WF (1996) Pathogen-induced systemic activation of a plant defensin gene in Arabidopsis follows a salicylic acid-independent pathway. Plant Cell 8:2309–2323
Sambrock J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Sanders PM, Lee PY, Biesgen C, Boone JD, Beals TP, Weiler EW, Goldberg RB (2000) The Arabidopsis DELAYED DEHISCENCE1 gene encodes an enzyme in the jasmonic acid synthesis pathway. Plant Cell 12:1041–1061
Schaller F (2001) Enzymes of the biosynthesis of octadecanoid-derived signalling molecules. J Exp Bot 52:11–23
Staswick PE, Su W, Howell SH (1992) Methyl jasmonate inhibition of root growth and induction of a leaf protein are decreased in an Arabidopsis thaliana mutant. Proc Natl Acad Sci USA 89:6837–6840
Staswick PE, Tiryaki I, Rowe ML (2002) Jasmonate response locus JAR1 and several related Arabidopsis genes encode enzymes of the firefly luciferase superfamily that shows activity on jasmonic, salicylic, and indole-3 acetic acids in an assay for adenylation. Plant Cell 14:1405–1415
Stelmach BA, Muller A, Hennig P, Gebhardt S, Schubert-Zsilavecz M, Weiler EW (2001) A novel class of oxylipins, sn1-O-(12-oxophytodienoyl)-sn2-O-(hexadecatrienoyl)-monogalactosyl diglyceride, from Arabidopsis thaliana. J Biol Chem 276:12832–8
Stintzi A, Browse J (2000) The Arabidopsis male-sterile mutant, opr3, lacks the 12-oxophytodienoic acid reductase required for jasmonate synthesis. Proc Natl Acad Sci USA 97:10625–10630
Stintzi A, Weber H, Reymond P, Browse J, Farmer EE (2001) Plant defense in the absence of jasmonic acid: The role of cyclopentenones. Proc Natl Acad Sci USA 98:12837–12842
Thomma BPHJ, Eggermont K, Penninckx, IAMA, Mauch-Mani B, Vogelsang R, Cammue BPA, Broekaert WF(1998) Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc Natl Acad Sci USA 95:15107–15111
Tiryaki I, Staswick PE (2002) An Arabidopsis mutant defective in jasmonate response is allelic to the auxin-signaling mutant axr1. Plant Physiol 130:887–94
Towbin H, Staehlin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedures and some applications. Proc Natl Acad Sci USA 76:4350–4354
Vick BA, Zimmerman DC (1984) Biosynthesis of jasmonic acid by several plant species. Plant Physiol 75:92–97
Weiler EW, Kutchan TM, Gorba T, Brodschelm W, Niesel U, Bublitz F (1994) The Pseudomonas phytotoxin coronatine mimics octadecanoid signaling molecules of higher plants. FEBS Lett 345:9–13
Xie D-X, Feys BF, James S, Nieto-Rostro M, Turner JG (1998) COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 280:1091–1094
Xu L, Liu F, Wang Z, Peng W, Huang R, Huang D, Xie D (2001) An Arabidopsis mutant cex1 exhibits constant accumulation of jasmonate-regulated AtVSP, Thi2.1 and PDF1.2. FEBS Lett 494:161–164
Zimmerman DC, Feng P (1978) Characterization of a prostaglandin-like metabolite of linolenic acid produced by a flaxseed extract. Lipids 13:313–316
Acknowledgements
This work was funded by the Deutsche Forschungsgemeinschaft, Bonn (SFB 480, TPA3) and by Fonds der Chemischen Industrie, Frankfurt a.M. (literature provision). The authors thank Dr. Dietmar Laudert, Bochum, for his logistic input and for generating the mutated seeds, Tülay Basöngen and Ina Schulze-Wiehenbrauck, for technical assistance and Dr. Axel Müller, Bochum, for help with the GC–MS/MS analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kubigsteltig, I.I., Weiler, E.W. Arabidopsis mutants affected in the transcriptional control of allene oxide synthase, the enzyme catalyzing the entrance step in octadecanoid biosynthesis. Planta 217, 748–757 (2003). https://doi.org/10.1007/s00425-003-1056-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-003-1056-x