Abstract
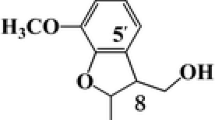
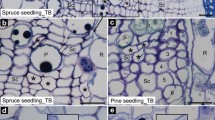
The lignification process in mature Norway spruce [Picea abies (L.) H. Karsten] xylem cell walls was studied using transmission electron microscopy (TEM)–immunogold detection with a polyclonal antibody raised against a specific lignin substructure, dibenzodioxocin. The study reveals for the first time the exact location of this abundant eight-ring structure in the cell wall layers of wood. Spruce wood samples were collected in Southern Finland at the time of active growth and lignification of the xylem cell walls. In very young tracheids where secondary cell wall layers were not yet formed, the presence of the dibenzodioxocin structure could not be shown at all. During secondary cell wall thickening, the dibenzodioxocin structure was more abundant in the secondary cell wall layers than in the middle lamella. The highest number of gold particles revealing dibenzodioxocin was in the S2+S3 layer. Statistically significant differences were found in the frequency of gold particles present in various cell wall layers. For comparison, wood sections were also cut with a cryomicrotome for light and fluorescence microscopy.



Similar content being viewed by others
Abbreviations
- BSA:
-
bovine serum albumin
- ELISA:
-
enzyme linked immunosorbent assay
- KLH:
-
keyhole limpet hemocyanin
- TEM:
-
transmission electron microscopy
References
Adler E, Gustafsson B (1963) Darstellung der threo- und erythro-Formen des D,L-Guajacyl-glycerins und des D,L-Veratrylglycerins. Acta Chem Scand 17:27–36
Ämmälahti E, Brunow G, Bardet M, Robert D, Kilpeläinen I (1999) Identification of side-chain structures in a poplar lignin using three-dimensional HMQC–HOHAHA NMR spectroscopy. J Agric Food Chem 46:5113–5117
Argyropoulos DS, Jurasek L, Kristofova L, Xia Z, Sun Y, Palus E (2002) Abundance and reactivity of dibenzodioxocins in softwood lignin. J Agric Food Chem 50:658–666
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Brunow G, Kilpeläinen I, Sipilä, J, Syrjänen K, Karhunen P, Setälä H, Rummakko P (1998) Oxidative coupling of phenols and the biosynthesis of lignin. In: Lewis NG, Sarkanen S (eds) Lignin and lignan biosynthesis. ACS Symp Ser, vol 697. American Chemical Society, Washington, pp 131–147
Davin LB, Lewis NG (2000) Dirigent proteins and dirigent sites explain the mystery of specificity of radical precursor coupling in lignan and lignin biosynthesis. Plant Physiol 123:453–462
Dixon RA, Chen F, Guo D, Parvathi K (2001) The biosynthesis of monolignols: a "metabolic grid", or independent pathways to guaiacyl and syringyl units? Phytochemistry 57:1069–1084
Fagerstedt K, Saranpää P, Piispanen R (1998) Peroxidase activity, isoenzymes and histological localization in sapwood and heartwood of Scots pine (Pinus sylvestris L.). J For Res 3:43–47
Fukushima K, Terashima N (1991) Heterogeneity in formation of lignin. XIV. Formation and structure of lignin in differentiating xylem of Ginkgo biloba. Holzforschung 45:87–94
Grünwald C, Ruel K, Joseleau J-P, Fladung M (2001) Morphology, wood structure and cell wall composition of rolC transgenic and non-transformed aspen trees. Trees 15:503–517
Joseleau J-P, Ruel K (1997) Study of lignification by noninvasive techniques in growing maize internodes. An investigation by Fourier transform Infrared, CP/MAS 13C NMR spectroscopy and immunocytochemical transmission electron microscopy. Plant Physiol 114:1123–1133
Karhunen P, Rummakko P, Sipilä S, Brunow G (1995) Dibenzodioxocins; a novel type of linkage in softwood lignins. Tetrahedron Lett 36:169–170
Karhunen P, Rummakko P, Pajunen A, Brunow G (1996) Synthesis and crystal structure determination of model compounds for the dibenzodioxocine structure occurring in wood lignins. J Chem Soc Perkin Trans 1:2303–2308
Lewis NG, Sarkanen S (1998) Lignin and lignan biosynthesis. ACS Symp Ser, vol 697. American Chemical Society, Washington
Monties B (1989) Lignins. In: Harborne JB (ed) Methods in plant biochemistry, vol 1, Plant phenolics. Academic Press, London, pp 113–157
Müsel G, Schindler T, Bergfeld R, Ruel K, Jacquet G, Lapierre C, Speth V, Schopfer P (1997) Structure and distribution of lignin in primary and secondary cell walls of maize coleoptiles analyzed by chemical and immunological probes. Planta 201:146–159
Nakatsubo F, Sato K, Higuchi T (1975) Synthesis of guaiacylglycerol-β-guaiacyl ether. Holzforschung 29:165–168
Quideau S, Ralph J (1994) A biomimetic route to lignin model compounds via silver (I) oxide oxidation. Holzforschung 48:12–22
Ruel K, Faix O, Joseleau J-P (1994) New immunogold probes for studying the distribution of the different lignin types during plant cell wall biogenesis. J Trace Microprobe Tech 12:247–265
Ruel K, Burlat V, Joseleau J-P (1999) Relationship between ultrastructural topochemistry of lignin and wood properties. IAWA J 20:203–211
Ruel K, Chabannes M, Boudet A-M, Legrand M, Joseleau J-P (2001) Reassessment of qualitative changes in lignification of transgenic tobacco plants and their impact on cell wall assembly. Phytochemistry 57:875–882
Ruel K, Montiel MD, Goujon T, Jouanin L, Burlat V, Joseleau J-P (2002) Interrelation between lignin deposition and polysaccharide matrices during the assembly of plant cell walls. Plant Biol 4:2–8
Saranpää P (1988) Plastids and glycolipids in the stemwood of Pinus sylvestris L. Trees 2:180–187
Savidge RA (2000) Biochemistry of seasonal cambial growth and wood formation—an overview of the challenges. In: Savidge RA, Barnett JR, Napier R (eds) Cell and molecular biology of wood formation. Experimental biology reviews. Bios, Oxford, pp 1–30
Srebotnik E, Messner K (1994) A simple method that uses differential staining and light microscopy to assess the selectivity of wood delignification by white rot fungi. Appl Environ Microbiol 60:1383–1386
Terashima N (1990) A new mechanism for formation of a structurally ordered protolignin macromolecule in the cell wall of tree xylem. J Pulp Paper Sci 16:150–155
Terashima N, Fukushima K, He LF, Takabe K (1993) Comprehensive model of the lignified plant cell wall. In: Jung HG, Buxton DR, Hatfield RD, Ralph J (eds) Forage cell wall structure and digestibility. American Society of Agronomy, Madison pp 247–269
Terashima N, Nakashima J, Takabe K (1998) Proposed structure for protolignin in plant cell walls. In: Lewis NG, Sarkanen S (eds) Lignin and lignan biosynthesis. ACS Symp Ser, vol 697. American Chemical Society, Washington, pp 180–193
Whetten R, Sederoff R (1995) Lignin biosynthesis. Plant Cell 7:1001–1013
Yoshinaga A, Fujita M, Saiki H (1997) Cellular distribution of guaiacyl and syringyl lignins within annual ring in oak wood. Mokuzai Gakkaishi 43:384–390
Acknowledgements
This investigation was funded by the Academy of Finland (grant no: 43091) and the National Technology Agency TEKES under The Finnish Forest Cluster Research Program WOOD WISDOM. This work is a part of the Center of Excellence in Plant Biology and Forest Biotechnology (project no. 164346) as granted by the Academy of Finland and the Ministry of Education of Finland. Wood samples were prepared and electron micrographs taken at the Electron Microscopy Unit of the Institute of Biotechnology, University of Helsinki.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kukkola, E.M., Koutaniemi, S., Gustafsson, M. et al. Localization of dibenzodioxocin substructures in lignifying Norway spruce xylem by transmission electron microscopy–immunogold labeling. Planta 217, 229–237 (2003). https://doi.org/10.1007/s00425-003-0983-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-003-0983-x