Abstract
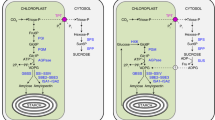
Transgenic plants of Arabidopsis thaliana Heynh., transformed with a bacterial β-glucuronidase (GUS) gene under the control of the promoter of the small subunit (ApS) of ADP-glucose pyrophosphorylase (AGPase), exhibited GUS staining in leaves (including stomata), stems, roots and flowers. Cross-sections of stems revealed GUS staining in protoxylem parenchyma, primary phloem and cortex. In young roots, the staining was found in the root tips, including the root cap, and in vascular tissue, while the older root–hypocotyl axis showed prominent staining in the secondary phloem and paratracheary parenchyma of secondary xylem. The GUS staining co-localized with ApS protein, as found by tissue printing using antibodies against ApS. Starch was found only in cell and tissue types exhibiting GUS staining and ApS labelling, but not in all of them. For example, starch was lacking in the xylem parenchyma and secondary phloem of the root–hypocotyl axis. Sucrose potently activated ApS gene expression in leaves of wild-type (wt) plants, and in transgenic seedlings grown on sucrose medium where GUS activity was quantified with 4-methylumbelliferyl-β-glucuronide as substrate. Okadaic acid, an inhibitor of protein phosphatases 1 and 2A, completely blocked expression of ApS in mature leaves of wt plants and prevented GUS staining in root tips and flowers of the transgenic plants, suggesting a similar signal transduction mechanism for ApS expression in various tissues. The data support the key role of AGPase in starch synthesis, but they also underlie the ubiquitous importance of the ApS gene for AGPase function in all organs/tissues of Arabidopsis.





Similar content being viewed by others
Abbreviations
- AGPase:
-
ADP-glucose pyrophosphorylase
- ApL:
-
large subunit of AGPase
- ApS:
-
small subunit of AGPase
- GUS:
-
β-glucuronidase
- MUG:
-
4-methylumbelliferyl-β-glucuronide
- OKA:
-
okadaic acid
- Pi :
-
inorganic phosphate
- PGA:
-
3-phosphoglyceric acid
References
ap Rees T (1992) Synthesis of storage starch. In: Pollock CR, Farrar JF, Gordon AJ (eds) Carbon partitioning within and between organisms. Bios, Oxford, pp 115–131
Baskin TI, Wilson JE (1997) Inhibitors of protein kinases and phosphatases alter root morphology and disorganize cortical microtubules. Plant Physiol 113:493–502
Bialojan C, Takai A (1988) Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases—specificity and kinetics. Biochem J 256:283–290
Caspar T (1994) Genetic dissection of the biosynthesis, degradation and biological functions of starch. In: Meyerowitz EM, Somerville CR (eds) Arabidopsis. Cold Spring Harbor Laboratory Press, New York, pp 913–936
Cassab GI (1992) Localisation of cell wall proteins. In: Reid PD, Pont-Resica RF (eds) Tissue printing. Academic Press, San Diego, pp 23–40
Chen BY, Janes HW (1998) Multiple forms of ADP-glucose pyrophosphorylase from tomato leaf. Physiol Plant 103:491–496
Choi SB, Kim KH, Kavakli IH, Lee SK, Okita TW (2001) Transcriptional expression characteristics and subcellular localization of ADP-glucose pyrophosphorylase in the oil plant Perilla frutescens. Plant Cell Physiol 42:146–153
Ciereszko I, Kleczkowski LA (2002) Glucose and mannose regulate the expression of a major sucrose synthase gene in Arabidopsis via hexokinase-dependent mechanisms. Plant Physiol Biochem 40:907–911
Ciereszko I, Johansson H, Hurry V, Kleczkowski LA (2001a) Phosphate status affects the gene expression, protein content and enzymatic activity of UDP-glucose pyrophosphorylase in wild-type and pho mutants of Arabidopsis. Planta 212:598–605
Ciereszko I, Johansson H, Kleczkowski LA (2001b) Sucrose and light regulation of a cold-inducible UDP-glucose pyrophosphorylase gene via a hexokinase-independent and abscisic acid-insensitive pathway in Arabidopsis. Biochem J 354:67–72
Déjardin A, Sokolov LN, Kleczkowski LA (1999) Sugar/osmoticum levels modulate differential abscisic acid-independent expression of two stress-responsive sucrose synthase genes in Arabidopsis. Biochem J 344:503–509
Denyer K, Dunlap F, Thorbjørnsen T, Keeling P, Smith AM (1996) The major form of ADP-glucose pyrophosphorylase in maize endosperm is extra-plastidial. Plant Physiol 112:779–885
Du Jardin P, Harvengt L, Kirsch F, Le VQ, NguyenQuoc B, Yelle S (1997) Sink-cell-specific activity of a potato ADP-glucose pyrophosphorylase B-subunit promoter in transgenic potato and tomato plants. Planta 203:133–139
Eimert K, Wang SM, Lue WI, Chen JC (1995) Monogenic recessive mutations causing both late floral initiation and excess starch accumulation in Arabidopsis. Plant Cell 7:1703–1712
Fritzius T, Aeschbacher R, Wiemken A, Wingler A (2001) Induction of ApL3 expression by trehalose complements the starch-deficient Arabidopsis mutant adg2-1 lacking ApL1, the large subunit of ADP-glucose pyrophosphorylase. Plant Physiol 126:883–889
Fujiki Y, Ito M, Nishida I, Watanabe A (2000) Multiple signalling pathways in gene expression during sugar starvation. Pharmacological analysis of din gene expression in suspension-cultured cells of Arabidopsis. Plant Physiol 124:1139–1147
Gómez-CasatiDF, Iglesias AA (2002) ADP-glucose pyrophosphorylase from wheat endosperm. Purification and characterization of an enzyme with novel regulatory properties. Planta 214:428–434
Hondred D, Walker JM, Mathews DE, Vierstra RD (1999) Use of ubiquitin fusions to augment protein expression in transgenic plants. Plant Physiol 119:713–724
Ingvardsen C, Veierskov B, Joshi PA (2001) Immunohistochemical localisation of ubiquitin and the proteasome in sunflower (Helianthus annuus cv. Giganteus). Planta 213:333–341
Jefferson RA (1987) Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep 5:387–405
Jefferson RA, Wilson KJ (1991) The GUS fusion system. Plant Mol Biol Manual B14:1–53
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions—β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901-3907
Kilian A, Kleinhofs A, Villand P, Thorbjørnsen T, Olsen O-A, Kleczkowski LA (1994) Mapping of ADP-glucose pyrophosphorylase genes from barley. Theor Appl Genet 87:869–871
Kleczkowski LA (1999) A phosphoglycerate to inorganic phosphate ratio is the major factor in controlling starch levels in chloroplasts via ADP-glucose pyrophosphorylase regulation. FEBS Lett 448:153–156
Kleczkowski LA, Villand P, Lüthi E, Olsen OA, Preiss J (1993a) Insensitivity of barley endosperm ADP-glucose pyrophosphorylase to 3-phosphoglycerate and orthophosphate regulation. Plant Physiol 101:179–186
Kleczkowski LA, Villand P, Preiss J, Olsen OA (1993b) Kinetic mechanism and regulation of ADP-glucose pyrophosphorylase from barley (Hordeum vulgare) leaves. J Biol Chem 268:6228–6233
Kleczkowski LA, Sokolov LN, Luo C, Villand P (1999) Molecular cloning and spatial expression of an ApL1 cDNA for the large subunit of ADP-glucose pyrophosphorylase from Arabidopsis thaliana. Z Naturforsch C 54: 353–358
Leidreiter K, Heineke D, Heldt HW, Müller-Röber B, Sonnewald U, Willmitzer L (1995) Leaf-specific antisense inhibition of starch biosynthesis in transgenic potato plants leads to an increase in photoassimilate export from source leaves during the light period. Plant Cell Physiol 36:615–624
Li X, Xing J, Gianfagna TJ, Janes HW (2002) Sucrose regulation of ADP-glucose pyrophosphorylase subunit genes transcript levels in leaves and fruits. Plant Sci 162:239–244
Luo C, Kleczkowski LA (1999) Expression of barley ADP-glucose pyrophosphorylase in Escherichia coli: processing and regulatory considerations. Phytochemistry 50:209–214
MacCleery SA, Kiss JZ (1999) Plastid sedimentation kinetics in roots of wild-type and starch-deficient mutants of Arabidopsis. Plant Physiol 120:183–192
Müller-Röber BT, Kossmann J, Hannah LC, Willmitzer L, Sonnewald U (1990) One of two different ADP-glucose pyrophosphorylase genes from potato responds strongly to elevated levels of sucrose. Mol Gen Genet 224:136–146
Müller-Röber B, Sonnewald U, Willmitzer L (1992) Inhibition of the ADP-glucose pyrophosphorylase in transgenic potatoes leads to sugar-storing tubers and influences tuber formation and expression of tuber storage protein genes. EMBO J 11:1229–1238
Müller-Röber B, Lacognata U, Sonnewald U, Willmitzer L (1994) A truncated version of an ADP-glucose pyrophosphorylase promoter from potato specifies guard cell-selective expression in transgenic plants. Plant Cell 6:601–612
Nakamura Y, Kawaguchi K (1992) Multiple forms of ADP-glucose pyrophosphorylase of rice endosperm. Physiol Plant 84:336–342
Nakata PA, Okita TW (1995) Differential regulation of ADP-glucose pyrophosphorylase in the sink and source tissues of potato. Plant Physiol 108:361–368
Nakata PA, Okita TW (1996) Cis-elements important for the expression of the ADP-glucose pyrophosphorylase small-subunit are located both upstream and downstream from its structural gene. Mol Gen Genet 250:581–592
Rashotte AM, DeLong A, Muday GK (2001) Genetic and chemical reductions in protein phosphatase activity alter auxin transport, gravity response, and lateral root growth. Plant Cell 13:1683–1697
Rook F, Corke F, Card R, Munz G, Smith C, Bevan MW (2001) Impaired sucrose-induction mutants reveal the modulation of sugar-induced starch biosynthetic gene expression by abscisic acid signalling. Plant J 26:421–433
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Smith RD, Wilson JE, Walker JC, Baskin TI (1994) Protein phosphatase inhibitors block root hair growth and alter cortical cell shape of Arabidopsis roots. Planta 194:516–524
Sokolov LN, Déjardin A, Kleczkowski LA (1998) Sugars and light/ dark exposure trigger differential regulation of ADP-glucose pyrophosphorylase genes in Arabidopsis thaliana. Biochem J 336:681–687
Takeda S, Mano S, Ohto M, Nakamura K (1994) Inhibitors of protein phosphatases 1 and 2A block the sugar-inducible gene expression in plants. Plant Physiol 106:567–574
Thorbjørnsen T, Villand P, Kleczkowski LA, Olsen OA (1996) A single gene encodes two different transcripts for the ADP-glucose pyrophosphorylase small subunit from barley (Hordeum vulgare). Biochem J 313:149–154
Villand P, Aalen R, Olsen OA, Lüthi E, Lönneborg A, Kleczkowski LA (1992) PCR amplification and sequences of cDNA clones for the small and large subunits of ADP-glucose pyrophosphorylase from barley tissues. Plant Mol Biol 19:381–389
Villand P, Olsen OA, Kleczkowski LA (1993) Molecular characterization of multiple cDNA clones of ADP-glucose pyrophosphorylase from Arabidopsis thaliana. Plant Mol Biol 23:1279–1284
Wang S-J, Yeh K-W, Tsai C-Y (2001) Regulation of starch granule-bound starch synthase I gene expression by circadian clock and sucrose in the source tissue of sweet potato. Plant Sci 161:635–644
Wang SM, Chu, B, Lue WL, Yu TS, Eimert K, Chen JC (1997) adg2-1 represents a missense mutation in the ADPG pyrophosphorylase large subunit gene of Arabidopsis thaliana. Plant J 11:1121–1126
Wang SM, Lue WL, Yu TS, Long JH, Wang CN, Eimert K, Chen J (1998) Characterization of ADG1, an Arabidopsis locus encoding for ADPG pyrophosphorylase small subunit, demonstrates that the presence of the small subunit is required for large subunit stability. Plant J 13:63–70
Wen FS, Zhu YM, Hawes MC (1999) Effect of pectin methylesterase gene expression on pea root development. Plant Cell 11:1129–1140
Acknowledgements
This work was supported, in part, by grants from the Swedish Natural Science Research Council, the Swedish Foundation For Strategic Research, and the Umeå biotechnology fund (to L.A.K.), as well as from the Foundation for Polish Science (supporting a postdoctoral scholarship for A.S.) and grant no. 6 P04C 01920 from the State Committee for Scientific Research (KBN, Poland, to I.C.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Siedlecka, A., Ciereszko, I., Mellerowicz, E. et al. The small subunit ADP-glucose pyrophosphorylase (ApS) promoter mediates okadaic acid-sensitive uidA expression in starch-synthesizing tissues and cells in Arabidopsis . Planta 217, 184–192 (2003). https://doi.org/10.1007/s00425-003-0982-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-003-0982-y