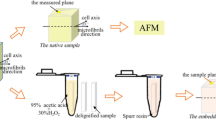
Abstract
Examination of angiosperm primary cell walls by transmission electron microscopy shows that they contain microfibrils that probably consist of cellulose microfibrils surrounded by associated non-cellulosic polysaccharides. Previous studies using solid-state 13C NMR spectroscopy have shown that the cellulose is all crystalline with crystallites of cross-sectional dimensions of 2–3 nm. However, it is not known if each microfibril contains only one, or more than one crystallite because there is no agreement about the dimensions of the microfibrils. Partially hydrated primary cell walls isolated from onion (Allium cepa L.) and Arabidopsis thaliana (L.) Heynh. were examined by atomic force microscopy and the microfibril diameters determined. The cell walls of both species contained tightly interwoven microfibrils of uniform diameter: 4.4±0.13 nm in the onion and 5.8±0.17 nm in A. thaliana. The effect was also examined of extracting the A. thaliana cell walls to remove pectic polysaccharides. The microfibrils in the extracted cell walls of A. thaliana were significantly narrower (3.2±0.13 nm) than those in untreated walls. The results are consistent with the microfibrils containing only one cellulose crystallite.



Similar content being viewed by others
Abbreviations
- AFM:
-
Atomic-force microscopy
- CDTA:
-
Trans-1,2-diaminocyclohexane N,N,N′,N′-tetraacetic acid
- TEM:
-
Transmission electron microscopy
References
Angiosperm Phylogeny Group (1998) An ordinal classification for the families of flowering plants. Ann Mo Bot Gard 85:531–553
Bacic A, Harris PJ, Stone BA (1988) Structure and function of plant cell walls. In: Preiss J (ed) The biochemistry of plants, vol 14. Carbohydrates. Academic Press, San Diego, pp 297–371
Carpita NC, Gibeaut DM (1993) Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J 3:1-30
Chanzy H, Imada K, Mollard A, Vuong R, Barnoud F (1979) Crystallographic aspects of sub-elementary cellulose fibrils occurring in the wall of rose cells cultured in vitro. Protoplasma 100:303–316
Cosgrove DJ (2001) Wall structure and wall loosening. A look backwards and forwards. Plant Physiol 125:131–134.
Davies LM, Harris PJ, Newman RH (2002) Molecular ordering of cellulose after extraction of polysaccharides from primary cell walls of Arabidopsis thaliana: a solid-state CP/MAS 13C NMR study. Carbohydr Res 337:587–593
Emons ACM (1988) Methods for visualizing cell-wall texture. Acta Bot Neerl 37:31–38
Engel A, Lyubchenko Y, Müller D (1999) Atomic force microscopy: a powerful tool to observe biomolecules at work. Trends Cell Biol 9:77–80
Feder N, O'Brien TP (1968) Plant microtechniques: some principles and new methods. Am J Bot 55:123–142
Franz GW, Blaschek W (1990) Cellulose. In: Dey PM, Harborne JB (eds) Methods in plant biochemistry, vol 2. Carbohydrates. Academic Press, London, pp 291–317
Fry SC (1989) The structure and functions of xyloglucan. J Exp Bot 40:1-11
Fujino T, Sone Y, Mitsuishi Y, Itoh T (2000) Characterization of cross-links between cellulose microfibrils, and their occurrence during elongation growth in pea epicotyl. Plant Cell Physiol 41:486–494
George EF, Sherrington PD (1984) Plant propagation by tissue culture. Exegetics, Eversley, UK
Ha M-A, Apperley DC, Evans BW, Huxham IM, Jardine WG, Vietor RJ, Reis D, Vian B, Jarvis MC (1998) Fine structure in cellulose microfibrils: NMR evidence from onion and quince. Plant J 16:183–190
Hanley SJ, Giasson J, Revol J-F, Gray DG (1992) Atomic force microscopy of cellulose microfibrils: comparison with transmission electron microscopy. Polymer 33:4639–4642
Harris PJ (1983) Cell walls. In: Hall JL, Moore AL (eds) Isolation of membranes and organelles from plant cells. Academic Press, London, pp 25–53
Harris PJ (2000) Compositions of monocotyledon cell walls: implications for biosystematics. In: Wilson KL, Morrison DA (eds) Monocots: systematics and evolution. CSIRO, Melbourne, pp 114–126
Harris PJ, Hartley RD, Lowry KH (1980) Phenolic constituents of mesophyll and non-mesophyll cell walls from leaf laminae of Lolium perenne. J Sci Food Agric 31:959–962
Kirby AR, Gunning AP, Waldron KW, Morris VJ, Ng A (1996) Visualization of plant cell walls by atomic force microscopy. Biophys J 70:1138–1143
Koh TH, Melton LD, Newman RH (1997) Solid-state 13C NMR characterization of cell walls of ripening strawberries. Can J Bot 75:1957–1964
Kuga S, Brown Jr RM (1991) Physical structure of cellulose microfibrils: implications for biogenesis. In: Haigler CH, Weimer PJ (eds) Biosynthesis and biodegradation of cellulose. Dekker, New York, pp 125–142
McCann MC, Wells B, Roberts K (1990) Direct visualisation of cross-links in the primary cell wall. J Cell Sci 96:323–334
Morris VJ, Kirby AR, Gunning AP (1999) Atomic force microscopy for biologists. Imperial College Press, London
Newman RH, Ha M-A, Melton LD (1994) Solid-state 13C NMR investigation of molecular ordering in the cellulose of apple cell walls. J Agric Food Chem 42:1402–1406
Newman RH, Davies LM, Harris PJ (1996) Solid-state 13C nuclear magnetic resonance characterization of cellulose in the cell walls of Arabidopsis thaliana leaves. Plant Physiol 111:475–485
Reis D, Vian B, Chanzy H, Roland JC (1991) Liquid crystal-type assembly of native cellulose-glucuronoxylans extacted from plant cell wall. Biol Cell 73:173–178
Roland JC, Vian B, Reis D (1975) Observations with cytochemistry and ultracryotomy on the fine structure of the expanding walls in actively elongating plant cells. J Cell Sci 19: 239–259
Smith BG, Harris PJ, Melton LD, Newman RH (1998) Crystalline cellulose in hydrated primary cell walls of three monocotyledons and one dicotyledon. Plant Cell Physiol 39:711–720
Thimm JC, Burritt DJ, Ducker WA, Melton LD (2000) Celery (Apium graveolens L.) parenchyma cell walls examined by atomic force microscopy: effect of dehydration on cellulose microfibrils. Planta 212:25–32
Thompson JE, Fry SC (2000) Evidence for covalent linkage between xyloglucan and acidic pectins in suspension-cultured rose cells. Planta 211:275–286
Wel NN van der, Putman CAJ, van Noort SJT, de Grooth BG, Emons AMC (1996) Atomic force microscopy of pollen grains, cellulose microfibrils, and protoplasts. Protoplasma 194:29–39
Acknowledgements
This research was supported by the Marsden Fund of the Royal Society of New Zealand (contract no. UOA 406). We thank the following from The University of Auckland: Mr K Goldie (School of Biological Sciences) and Dr R Haverkamp (School of Engineering) for assistance with the AFM; and Associate Prof. B McCardle (Statistics Department) for statistical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Davies, L.M., Harris, P.J. Atomic force microscopy of microfibrils in primary cell walls. Planta 217, 283–289 (2003). https://doi.org/10.1007/s00425-003-0979-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-003-0979-6