Abstract
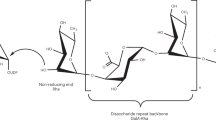
We investigated a galactosyltransferase (GalT) involved in the synthesis of the carbohydrate portion of arabinogalactan-proteins (AGPs), which consist of a β-(1→3)-galactan backbone from which consecutive (1→6)-linked β-Galp residues branch off. A membrane preparation from 6-day-old primary roots of radish (Raphanus sativus L.) transferred [14C]Gal from UDP-[14C]Gal onto a β-(1→3)-galactan exogenous acceptor. The reaction occurred maximally at pH 5.9–6.3 and 30 °C in the presence of 15 mM Mn2+ and 0.75% Triton X-100. The apparent K m and V max values for UDP-Gal were 0.41 mM and 1,000 pmol min−1 (mg protein)−1, respectively. The reaction with β-(1→3)-galactan showed a bi-phasic kinetic character with K m values of 0.43 and 2.8 mg ml−1. β-(1→3)-Galactooligomers were good acceptors and enzyme activity increased with increasing polymerization of Gal residues. In contrast, the enzyme was less efficient on β-(1→6)-oligomers. The transfer reaction for an AGP from radish mature roots was negligible but could be increased by prior enzymatic or chemical removal of α-l-arabinofuranose (α-l-Araf) residues or both α-l-Araf residues and (1→6)-linked β-Gal side chains. Digestion of radiolabeled products formed from β-(1→3)-galactan and the modified AGP with exo-β-(1→3)-galactanase released mainly radioactive β-(1→6)-galactobiose, indicating that the transfer of [14C]Gal occurred preferentially onto consecutive (1→3)-linked β-Gal chains through β-(1→6)-linkages, resulting in the formation of single branching points. The enzyme produced mainly a branched tetrasaccharide, Galβ(1→3)[Galβ(1→6)] Galβ(1→3)Gal, from β-(1→3)-galactotriose by incubation with UDP-Gal, confirming the preferential formation of the branching linkage. Localization of the GalT in the Golgi apparatus was revealed on a sucrose density gradient. The membrane preparation also incorporated [14C]Gal into β-(1→4)-galactan, indicating that the membranes contained different types of GalT isoform catalyzing the synthesis of different types of galactosidic linkage.






Similar content being viewed by others
Abbreviations
- ABEE:
-
p-aminobenzoic acid ethyl ester
- AGP:
-
arabinogalactan-protein
- Araf :
-
arabinofuranose
- DP:
-
degree of polymerization
- GalT:
-
galactosyltransferase
- GlcA:
-
glucuronic acid
- GlcNAc:
-
N-acetylglucosamine
- MALDI-TOF-MS:
-
matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
- 4-O-methyl-GlcA:
-
4-O-methyl-glucuronic acid
- PNP:
-
p-nitrophenyl
References
Albersheim P, Nevins DJ, English PD, Karr A (1967) A method for the analysis of sugars in plant cell-wall polysaccharides by gas liquid chromatography. Carbohydr Res 5:340–345
Aspinall GO, Auret BJ, Hirst EL (1958a) Gum ghatti (Indian gum). Part Ⅲ. Neutral oligosaccharides found on partial acid hydrolysis of the gum. J Chem Soc 4408–4414
Aspinall GO, Hirst EL, Ramstad E (1958b) The constitution of larch ε-galactan. J Chem Soc 593–601
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brickell LS, Reid JSG (1996) Biosynthesis in vitro of pectic (1→4)-β-d-galactan. In: Visser J, Voragen AGJ (eds) Pectins and pectinases. Elsevier, Amsterdam, pp 127–134
Caspar Y, Johnson KL, McKenna JA, Bacic A, Schultz CJ (2001) The complex structures of arabinogalactan-proteins and the journey towards understanding function. Plant Mol Biol 47:161–176
Clarke AE, Anderson RL, Stone BA (1979) Form and function of arabinogalactans and arabinogalactan-proteins. Phytochemistry 18:521–540
Fincher GB, Stone BA, Clarke AE (1983) Arabinogalactan-proteins: structure, biosynthesis and function. Annu Rev Plant Physiol 34:47–70
Geshi N, Jørgensen B, Scheller HV, Ulvskov P (2000) In vitro biosynthesis of 1,4-β-galactan attached to rhamnogalacturonan Ⅰ. Planta 210:622–629
Gibeaut DM, Carpita NC (1991) Tracing cell wall biogenesis in intact cells and plants. Selective turnover and alteration of soluble and cell wall polysaccharides in grasses. Plant Physiol 97:551–561
Goubet F, Morvan C (1993) Evidence for several galactan synthases in flax (Linum usitatissimum L.) suspension-cultured cells. Plant Cell Physiol 34:1297–1303
Goubet F, Morvan C (1994) Synthesis of cell wall galactans from flax (Linum usitatissimum L.) suspension-cultured cells. Plant Cell Physiol 35:719–727
Goudsmit EM, Ketchum PA, Grossens MK, Blake DA (1989) Biosynthesis of galactogen: identification of a β-(1→6)-d-galactosyltransferase in Helix pomatia albumen glands. Biochim Biophys Acta 992:289–297
Hakomori S (1964) A rapid permethylation of glycolipid, and polysaccharide catalyzed by methylsulfinyl carbanion in dimethyl sulfoxide. J Biochem 55:205–208
Hashimoto Y (2000) Structure and biosynthesis of l-fucosylated arabinogalactan-proteins in cruciferous plants. In: Nothnagel EA et al. (eds) Cell and developmental biology of arabinogalactan-proteins. Kluwer/Plenum, New York, pp 51–60
Hayashi T, Maclachlan G (1984) Glycolipids and glycoproteins formed from UDP-galactose by pea membranes. Phytochemistry 23:487–492
Ishikawa M, Kuroyama H, Takeuchi Y, Tsumuraya Y (2000) Characterization of pectin methyltransferase from soybean hypocotyls. Planta 210:782–791
Joziasse DH, Damen HCM, De Jong-Brink M, Edzes HT, Van den Eijnden DH (1987) Identification of UDP-Gal:β-galactoside β1→3-galactosyltransferase in the albumen gland of the snail Lymnaea stagnalis. FEBS Lett 221:139–144
Kooiman P (1961) The constitution of Tamarindus-amyloid. Rec Trav Chim Pays-Bas 80:849–865
Kováč P (1985) Systematic chemical synthesis of (1→6)-β-d-galacto-oligosaccharides and related compounds. Carbohydr Res 144:C12-C14
Kováč P, Glaudemans CPJ, Taylor RB (1985) An efficient, unambiguous synthesis of methyl 3-O-β-d-galactopyranosyl-β-d-galactopyranoside. Further studies on the specificity of antigalactopyranan monoclonal antibodies. Carbohydr Res 142:158–164
Kuroyama H, Tsumuraya Y (2001) A xylosyltransferase that synthesizes β-(1→4)-xylans in wheat (Triticum aestivum L.) seedlings. Planta 213:231–240
Mascara T, Fincher GB (1982) Biosynthesis of arabinogalactan-protein in Lolium multiflorum (ryegrass) endosperm cells. Ⅱ. In vitro incorporation of galactosyl residues from UDPgalactose into polymeric products. Aust J Plant Physiol 9:31–45
Matsuura F, Imaoka A (1988) Chromatographic separation of asparagine-linked oligosaccharides labeled with an ultraviolet-absorbing compound, p-aminobenzoic acid ethyl ester. Glycoconj J 5:13–26
McNab JM, Villemez CL, Albersheim P (1968) Biosynthesis of galactan by a particulate enzyme preparation from Phaseolus aureus seedlings. Biochem J 106:355–360
Misawa H, Tsumuraya Y, Kaneko Y, Hashimoto Y (1996) α-l-Fucosyltransferases from radish primary roots. Plant Physiol 110:665–673
Nakano H, Takenishi S, Watanabe Y (1985) Purification and properties of two galactanases from Penicillium citrinum. Agric Biol Chem 49:3445–3454
Nothnagel EA (1997) Proteoglycans and related components in plant cells. Int Rev Cytol 174:195–291
O'Neill M, Albersheim P, Darvill A (1990) The pectic polysaccharides of primary cell walls. In: Dey PM (ed) Methods in plant biochemistry, vol 2. Academic Press, London, pp 415–491
Panayotatos N, Villemez CL (1973) The formation of a β-(1→4)-d-galactan chain catalysed by a Phaseolus aureus enzyme. Biochem J 133:263–271
Peugnet I, Goubet F, Bruyant-Vannir MP, Thoiron B, Morvan C, Schols HA, Voragen AGJ (2001) Solubilization of rhamnogalacturonan I galactosyltransferases from membranes of a flax cell suspension. Planta 213:435–445
Ridley BL, O'Neill MA, Mohnen D (2001) Pectins: structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 57:929–967
Roy A, Manjula BN, Glaudemans CPJ (1981) The interaction of two polysaccharides containing β1,6-linked galactopyranosyl residues with two monoclonal antigalactan immunoglobulin Fab′ fragments. Mol Immunol 18:79–84
Schibeci A, Pnjak A, Fincher GB (1984) Biosynthesis of arabinogalactan-protein in Lolium multiflorum (Italian ryegrass) endosperm cells. Subcellular distribution of galactosyltransferases. Biochem J 218:633–636
Sekimata M, Ogura K, Tsumuraya Y, Hashimoto Y, Yamamoto S (1989) A β-galactosidase from radish (Raphanus sativus L.) seedlings. Plant Physiol 90:567–574
Stangier K, Lüttge H, Thiem JE, Bretting H (1995) Biosynthesis of the storage polysaccharide from the snail Biomphalaria glabrata, identification and specificity of a branching β1→6 galactosyltransferase. J Comp Physiol B 165:278–290
Takeuchi Y, Komamine A (1980) Turnover of cell wall polysaccharides of a Vinca rosea suspension culture. Ⅲ. Turnover of arabinogalactan. Physiol Plant 50:113–118
Tsumuraya Y, Hashimoto Y, Yamamoto S, Shibuya N (1984) Structure of l-arabino-d-galactan-containing glycoproteins from radish leaves. Carbohydr Res 134:215–228
Tsumuraya Y, Hashimoto Y, Yamamoto S (1987) An l-arabino-d-galactan and an l-arabino-d-galactan-containing proteoglycan from radish (Raphanus sativus) seeds. Carbohydr Res 161:113–126
Tsumuraya Y, Mochizuki N, Hashimoto Y, Kováč P (1990) Purification of an exo-β-(1→3)-d-galactanase of Irpex lacteus (Polyporus tulipiferae) and its action on arabinogalactan-proteins. J Biol Chem 265:7207–7215
Tsumuraya Y, Ogura K, Hashimoto Y, Mukoyama H, Yamamoto S (1988) Arabinogalactan-proteins from primary and mature roots of radish (Raphanus sativus L.) Plant Physiol 86:155–160
Author information
Authors and Affiliations
Corresponding author
Additional information
Sugars described in this paper belong to the d-series unless otherwise noted
Rights and permissions
About this article
Cite this article
Kato, H., Takeuchi, Y., Tsumuraya, Y. et al. In vitro biosynthesis of galactans by membrane-bound galactosyltransferase from radish (Raphanus sativus L.) seedlings. Planta 217, 271–282 (2003). https://doi.org/10.1007/s00425-003-0978-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-003-0978-7