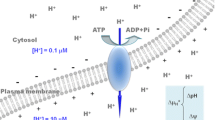
Abstract.
The plant plasma membrane H+-ATPase contains a C-terminal autoinhibitory domain whose displacement from the catalytic site is caused by binding of regulatory 14-3-3 proteins. Members of the highly conserved 14-3-3 family bind their individual target proteins in a sequence-specific and phosphorylation-dependent manner within a central groove, the latter characterized by the presence of highly invariant residues. However, an atypical binding site for 14-3-3s within the H+-ATPase has been identified that does not resemble any other 14-3-3 binding motif. Combination of site-directed mutagenesis with glutathione S-transferase pull-down assays points to the importance of the central 14-3-3 groove for the interaction with the apparently unique site of the H+-ATPase. Furthermore, a 14-3-3 dimer is essential for binding such unusual motif.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Jaspert, N., Oecking, C. Regulatory 14-3-3 proteins bind the atypical motif within the C terminus of the plant plasma membrane H+-ATPase via their typical amphipathic groove. Planta 216, 136–139 (2002). https://doi.org/10.1007/s00425-002-0915-1
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00425-002-0915-1