Abstract
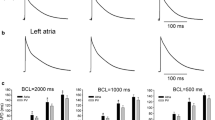
We used the whole-cell patch-clamp technique and monitoring of Fura-2 fluorescence to investigate the voltage dependence of the L-type Ca current (I Ca,L) and intracellular Ca (Cai) transient in rabbit atrial myocytes at 37°C. Imaging the atrial cell membrane with Di-4-ANNEPS showed (in contrast to ventricular cells) that atrial cells had very few transverse tubules. We measured I Ca,L using a Cs-based internal dialysis solution to eliminate interfering K currents. The voltage dependence of peak I Ca,L amplitude was bell-shaped: I Ca,L was maximal at +10 mV and declined at more negative and positive potentials. For measuring the Fura-2 (Cai) transient, we used a K-based internal dialysis solution to preserve normal excitation–contraction coupling. Ryanodine (20 μM) plus thapsigargin (2 μM) (blockers of the sarcoplasmic reticulum, SR) abolished the phasic component of the Fura-2 transient (n = 5), demonstrating that the phasic Fura-2 transient provided an index of the magnitude of SR release. The Fura-2 transient also showed bell-shaped voltage dependence, but this was different from that for I Ca,L. The Fura-2 transient peaked at +30 mV and partially declined at more positive potentials; but at potentials where inward I Ca,L was small (if not absent), the phasic Fura-2 transient still attained a significant amplitude. We used a rapid application of nifedipine (32 μM), and of nifedipine plus 5 mM Ni, to assess the ability of I Ca,L and reverse-mode Na-Ca exchange to trigger SR Ca release. With test pulses to +10 mV and +60 mV, a rapid switch to nifedipine (which blocked I Ca,L) produced no significant reduction in phasic Fura-2 transient amplitude. This suggests that in the absence of I Ca,L, another mechanism was able to trigger SR release. With pulses to +10 and +60 mV, a single beat switch to nifedipine plus 5 mM Ni almost completely abolished the phasic transient. Since 5 mM Ni inhibits Na-Ca exchange, this suggests that, in the absence of I Ca,L, trigger Ca entry via reverse Na-Ca exchange was able to activate SR Ca release in atrial cells at 37°C. The mechanisms underlying the Fura-2 transient in atrial cells, and differences with pre-existing data from rabbit ventricular cells, are discussed.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 24 September 1996 / Received after revision and accepted: 19 December 1996
Rights and permissions
About this article
Cite this article
Mitcheson, J., Hancox, J. & Levi, A. Voltage dependence of the Fura-2 transient in rabbit left atrial myocytes at 37°C. Pflügers Arch 433, 817–826 (1997). https://doi.org/10.1007/s004240050350
Issue Date:
DOI: https://doi.org/10.1007/s004240050350