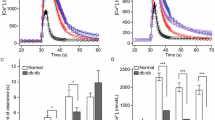
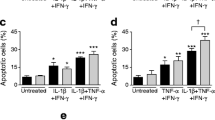
Abstract
Pancreatic beta cells utilize Ca2+ to secrete insulin in response to glucose. The glucose-dependent increase in cytosolic Ca2+ concentration ([Ca2+]C) activates a series of insulin secretory machinery in pancreatic beta cells. Therefore, the amount of insulin secreted in response to glucose is determined in a [Ca2+]C-dependent manner, at least within a moderate range. However, the demand for insulin secretion may surpass the capability of beta cells. Abnormal elevation of [Ca2+]C levels beyond the beta-cell endurance capacity can damage them by inducing endoplasmic reticulum (ER) stress and cell death programs such as apoptosis. Therefore, while Ca2+ is essential for the insulin secretory functions of beta cells, it could affect their survival at pathologically higher levels. Because an increase in beta-cell [Ca2+]C is inevitable under certain hazardous conditions, understanding the regulatory mechanism for [Ca2+]C is important. Therefore, this review discusses beta-cell function, survival, ER stress, and apoptosis associated with intracellular and ER Ca2+ homeostasis.




Similar content being viewed by others
Data availability
Not applicable.
Abbreviations
- ATP:
-
Adenosine triphosphate
- AMP:
-
Adenosine monophosphate
- BiPS:
-
Binding immunoglobulin protein
- cAMP:
-
Cyclic AMP
- CRE:
-
CAMP-response element
- CREB:
-
CAMP-response element binding protein
- CRAC:
-
Ca2+ release–activated Ca2+
- EPAC:
-
Exchange protein directly activated by cAMP
- eIF2α:
-
Eukaryotic initiation factor 2α
- ER:
-
Endoplasmic reticulum
- ERAD:
-
ER-associated protein degradation
- GDP:
-
Guanosine diphosphate
- GTP:
-
Guanosine triphosphate
- GEF:
-
Guanine nucleotide exchange factor
- GLUT:
-
Glucose transporters
- GSIS:
-
Glucose-stimulated insulin secretion
- GPCRs:
-
Guanine-nucleotide-binding regulatory protein (G protein)-coupled receptors
- GLP:
-
Glucagon-like peptide
- GIP:
-
Glucose-dependent insulinotropic polypeptide
- GRP:
-
Glucose-regulated protein
- HOMA:
-
Homeostasis model assessment
- HSPA:
-
Heat shock 70 kDa protein
- Kir:
-
Inwardly-rectifying potassium channels
- IICR:
-
IP3-induced Ca2+ release
- IRE1:
-
Inositol-requiring 1
- JNK:
-
C-Jun N-terminal kinase
- KATP :
-
ATP-sensitive K+
- KV :
-
Voltage-dependent K+
- MA:
-
Malate-aspartate
- PERK:
-
Protein kinase RNA-like ER kinase
- Pyk:
-
Protein tyrosine kinase
- PHHI:
-
Persistent hyperinsulinemic hypoglycemia in infants
- PKA:
-
Protein kinase A
- ROS:
-
Reactive oxygen species
- RyR:
-
Ryanodine receptors
- RAP:
-
Ras-related protein
- SUR:
-
Sulfonylurea receptors
- SERCA:
-
Sarco/-endoplasmic reticulum Ca2+ ATPase
- SOCE:
-
Store-operated calcium entry
- Stim:
-
Stromal interaction molecule
- SARAF:
-
SOCE-associated regulatory factor
- TRP:
-
Transient receptor potential
- UPR:
-
Unfolded protein response
- VDCC:
-
Voltage-dependent Ca2+ channels
- WFS:
-
Wolfram syndrome
References
Ajoolabady A, Lindholm D, Ren J, Pratico D (2022) ER stress and UPR in Alzheimer’s disease: mechanisms, pathogenesis, treatments. Cell Death Dis 13(8):706. https://doi.org/10.1038/s41419-022-05153-5
Ansar MM, Ansari M (2006) Nitric oxide involvement in pancreatic beta cell apoptosis by glibenclamide. Nitric Oxide 14(1):39–44. https://doi.org/10.1016/j.niox.2005.09.002
Ashcroft FM (2005) ATP-sensitive potassium channelopathies: focus on insulin secretion. J Clin Invest 115(8):2047–2058. https://doi.org/10.1172/JCI25495
Ashcroft FM, Gribble FM (2000) New windows on the mechanism of action of KATP channel openers. Trends Pharmacol Sci 21(11):439–445. https://doi.org/10.1016/s0165-6147(00)01563-7
Baev AY, Vinkurov AY, Novikova IN, Dremin VV, Potapova EV, Abramov AY (2022) Interaction of mitochondrial calcium and ros in neurodegeneration. Cells 11(4):706. https://doi.org/10.3390/cells11040706
Ballinger SW, Shoffner JM, Hedaya EV, Trounce I, Polak MA, Koontz DA, Wallace DC (1992) Maternally transmitted diabetes and deafness associated with a 10.4 kb mitochondrial DNA deletion. Nat Genet 1(1):11–15. https://doi.org/10.1038/ng0492-11
Chen C, Cohrs CM, Stertmann J, Bozsak R, Speier S (2017) Human beta cell mass and function in diabetes: recent advances in knowledge and technologies to understand disease pathogenesis. Mol Metab 6(9):943–957. https://doi.org/10.1016/j.molmet.2017.06.019
Cnop M, Toivonen S, Igoillo-Esteve M, Salpea P (2017) Endoplasmic reticulum stress and eIF2α phosphorylation: the achilles heel of pancreatic β-cells. Mol Metab 6(9):1024–1039. https://doi.org/10.1016/j.molmet.2017.06.001
Collier JJ, Burke SJ, Eisenhauer ME, Lu D, Sapp RC, Frydman CJ, Campagna SR (2011) Pancreatic β-cell death in response to pro-inflammatory cytokines is distinct from genuine apoptosis. Plos One 6(7):e22485. https://doi.org/10.1371/journal.pone.0022485
Daverkausen-Fischer L, Prols F (2022) Regulation of calcium homeostasis and flux between the endoplasmic reticulum and the cytosol. J Biol Chem 298(7):102061. https://doi.org/10.1016/j.jbc.2022.102061
Denmeade SR, Isaacs JT (2005) The SERCA pump as a therapeutic target: making a “smart bomb” for prostate cancer. Cancer Biol Ther 4(1):14–22. https://doi.org/10.4161/cbt.4.1.1505
Eizirik DL, Cnop M (2010) ER stress in pancreatic beta cells: the thin red line between adaption and failure. Sci Signal 3:pe7. https://doi.org/10.1126/scisignal.3110pe7
Eizirik DL, Cardozo AK, Cnop M (2008) The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev 29(1):42–61. https://doi.org/10.1210/er.2007-0015
Fonseca SG, Gromada J, Urano F (2011) Endoplasmic reticulum stress and pancreatic β-cell death. Trends Endocrinol Metab 22(7):266–274. https://doi.org/10.1016/j.tem.2011.02.008
Goodge KA, Hutton JC (2000) Translational regulation of proinsulin biosynthesis and proinsulin conversion in the pancreatic beta-cell. Semin Cell Dev Biol 11(4):235–242. https://doi.org/10.1006/scdb.2000.0172
Gwiazda KS, Yang TL, Lin Y, Johnson JD (2009) Effects of palmitate on ER and cytosolic homeostasis in beta-cells. Am J Physiol Endorinol Metab 296(4):E690-701. https://doi.org/10.1152/ajpendo.90525.2008
Halls ML, Cooper DM (2011) Regulation by Ca2+ signaling pathways of adenylyl cyclases. Cold Spring Harb Perspect Biol 3(2):a004143. https://doi.org/10.1101/cshperspect.a004143
Han J, Back SH, Hur J, Lin YH, Gildersleeve R, Shan J, Yuan CL, Krokowski D, Wang S, Hatzoglou M, Kilberg MS, Sartor MA, Kaufman RJ (2013) ER stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol 15(5):481–490. https://doi.org/10.1038/ncb2738
Harraz OF, Altier C (2014) Stim1-mediated bidirectional regulation of Ca2+ entry through voltage-gated calcium channels (VFCC) and calcium-release activated channels (CRAC). Front Cell Neurosci 8:43. https://doi.org/10.3389/fncel.2014.00043
Henquin JC (2000) Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes 49(11):1751–1760. https://doi.org/10.2337/diabetes.49.11.1751
Holst JJ (2007) The physiology of glucagon-like peptide 1. Physiol Rev 87(4):1409–1439. https://doi.org/10.1152/physrev.00034.2006
Hou JC, Min L, Pessin JE (2009) Insulin granule biogenesis, trafficking and exocytosis. Vitam Horm 80:473–506. https://doi.org/10.1016/S0083-6729(08)00616-X
Inaishi J, Saisho Y, Sato S, Kou K, Murakami R, Watanabe Y, Kitago M, Kitagawa Y, Yamada T, Itoh H (2016) Effects of obesity and diabetes on α- and β-cell mass in surgically resected human pancreas. J Clin Endocrinol Metab 101(7):2874–2882. https://doi.org/10.1210/jc.2016-1374
Jezek P, Jaburek M, Plecita-Hlavata L (2019) Contribution of oxidative stress and impaired biogenesis of pancreatic β-cells to type 2 diabetes. Antioxid Redox Signal 31(10):722–751. https://doi.org/10.1089/ars.2018.7656
Kane C, Shepherd RM, Squires PE, Johnson PR, James RF, Milla PJ, Aynsley-Green A, Lindley KJ, Dunne MJ (1996) Loss of functional KATP channels in pancreatic beta-cells causes persistent hyperinsulinemic hypoglycemia of infancy. Nat Med 2(12):1344–1347. https://doi.org/10.1038/nm1296-1344
Kappel S, Borgstrom A, Stoklosa P, Dorr K, Peinelt C (2019) Store-operated calcium entry in disease: beyond STIM/ORAI expression levels. Semin Cell Dev Biol 94:66–73. https://doi.org/10.1016/j.semcdb.2019.01.003
Karunakaran U, Kim HJ, Kim JY, Lee IK (2012) Guards and culprits in the endoplasmic reticulum: glucolipotoxicity and β-cell failure in type II diabetes. Exp Diabetes Res 2012:639762. https://doi.org/10.1155/2012/639762
Kataoka HU, Noguchi H (2013) ER stress and β-cell pathogenesis of type1 and type2 diabetes and islet transplantation. Cell Med 5(2–3):53–57. https://doi.org/10.3727/215517913X666512
Khosravi-Far R, Esposti MD (2004) Death receptor signals to mitochondria. Cancer Biol Ther 3(11):1051–1057. https://doi.org/10.4161/cbt.3.11.1173
Kim W, Egan JM (2008) The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev 60(4):470–512. https://doi.org/10.1124/pr.108.000604
Leech CA, Chepurny OG, Halz GG (2010) Epac2-dependent rap1 activation and the control of islet insulin secretion by glucagon-like peptide-1. Vitam Horm 84:279–302. https://doi.org/10.1016/B978-0-12-381517-0.00010-2
Leibowitz G, Glaser B, Higazi AA, Salameh M, Cerasi E, Landau H (1995) Hyperinsulinemic hypoglycemia of infancy (nesidioblastosis) in clinical remission: high incidence of diabetes mellitus and perisistent beta-cell dysfunction at long-term follow-up. J Clin Endocrinol Metab 80(2):386–392. https://doi.org/10.1210/jcem.80.2.7852494
Lenaire K, Schuit F (2012) Integrating insulin secretion and ER stress in pancreatic -cells. Nat Cell Biol 14(10):979–981. https://doi.org/10.1038/ncb2594
Liao Y, Erxleben C, Abramowitz J, Flockerzi V, Zhu MX, Armstrong DL, Birnbaumer L (2008) Functional interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model of SOCE/Icrac channels. Proc Natl Acad Sci U S A 105(8):2895–2900. https://doi.org/10.1073/pnas.0712288105
Lin JH, Walter P, Yen TS (2008) Endoplasmic reticulum stress in disease pathogenesis. Annu Rev Pathol 3:399–425. https://doi.org/10.1146/annurev.pathmechdis.3.121806.151434
Lindholm D, Wootz H, Korhonon L (2006) ER stress and neurodegenerative diseases. Cell Death Differ 13(3):385–392. https://doi.org/10.1038/sj.cdd.4401778
Liu K, Tsung K, Attenello FJ (2020) Characterizing cell stress and GRP78 in glioma to enchance tumor treatment. Front Oncol 10:608911. https://doi.org/10.3389/fonc.2020.608911
Lopez JJ, Albarran L, Gomez LJ, Smani T, Salido GM (1863) Rosado JA (2016) Molecular modulators of store-operated calcium entry. Biochim Biophys Acta 8:2037–2043. https://doi.org/10.1016/j.bbamcr.2016.04.024
Lopez E, Frischauf I, Jardin I, Derler I, Muik M, Cantonero C, Salido GM, Smani T, Rosado JA, Redondo PC (2019) STIM1 phosphorylation at Y316 modulates its interaction with SARAF and the activation of SOCE and ICRAC. J Cell Sci 132(10):jcs226019. https://doi.org/10.1242/jcs.226019
Lopez JJ, Jardin I, Sanchez-Collado J, Salido GM, Smani T, Rosado JA (2020) TRPC channels in the SOCE scenario. Cells 9(1):126. https://doi.org/10.3390/cells9010126
Luciani DS, Gwiazda KS, Yang TL, Kalynyak TB, Bychkivska Y, Frey MH, Jeffrey KD, Sampaio AV, Underhill TM, Johnson JD (2009) Roles of IP3R and RyR Ca2+ channels in endoplasmic reticulum stress and beta-cell death. Diabetes 58(2):422–432. https://doi.org/10.2337/db07-1762
Ma ZA, Zhao Z, Turk J (2012) Mitochondrial dysfunction and β-cell failure in type 2 diabetes mellitus. Exp Diabetes Res 2012:703538. https://doi.org/10.1155/2012/703538
Maliougina M, El Hiani Y (2023) TRPM2: bridging calcium and ROS signaling pathways-implications for human diseases. Front Physiol 14:1217828. https://doi.org/10.3389/fphys.2023.1217828
Marrif HI, Al-Sunousi SI (2016) Pancreatic β-cell mass death. Front Pharmacol 7:83. https://doi.org/10.3389/fphar.2016.00083
Moccia F, Fiorio Pla A, Lim D, Lodola F, Gerbino A (2023) Intracellular Ca2+ signaling: unexpected new roles for the usual suspect. Front Physiol 14:1210085. https://doi.org/10.3389/fphys.2023.1210085
Nagao M, Lagerstedt JO, Eliasson L (2022) Secretory granule exocytosis and its amplification by cAMP in pancreatic β-cells. Diabetol Int 13(3):471–479. https://doi.org/10.1007/s13340-022-00580-3
Nakamura RK, Mann LS, Lindner MD, Braithwaite J, Chen MC, Vancea A, Byrnes N, Durrant V, Reed B (2021) An experimental test of the effects of redacting grant applicant identifiers on peer review outcomes. Elife 10:e71368. https://doi.org/10.7554/eLife.71368
Oh YS, Bae GD, Baek DJ, Park EY, Jun HS (2018) Fatty acid-induced lipotoxicity in pancreatic beta-cells during development of type 2 diabetes. Front Endocrinol (Lausanne) 9:384. https://doi.org/10.3389/fendo.2018.00384
Parekh AB (2003) Store-operated Ca2+ entry: dynamic inteplay between endoplasmic reticulum, mitochondria and plasma membrane. J physiol 547(Pt 2):333–348. https://doi.org/10.1113/jphysiol.2002.034140
Perez-Zoghbi JF, Karner C, Ito S, Shepherd M, Alrashdan Y, Sanderson MJ (2009) lon channel regulation of intracellular calcium and airway smooth muscle function. Pulm Pharmacol Ther 22(5):388–397. https://doi.org/10.1016/j.popt.2008.09.006
Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R (2008) Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene 27(50):6407–6418. https://doi.org/10.1038/onc.2008.308
Putney JW (2011) The physiological function of store-operated calcium entry. Neurochem Res 36(7):1157–1165. https://doi.org/10.1007/s11064-010-0383-0
Rao RV, Ellerby HM, Bredesen DE (2004) Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ 11(4):372–380. https://doi.org/10.1038/sj.cdd.4401378
Rashid HO, Yadav RK, Kim HR, Chae HJ (2015) ER stress: autophagy induction, inhibition and selection. Autophagy 11(11):1956–1977. https://doi.org/10.1080/15548627.2015.1091141
Roder PV, Wu B, Liu Y, Han W (2016) Pancreatic regulation of glucose homeostasis. Exp Mol Med 48(3):e219. https://doi.org/10.1038/emm.2016.6
Sabatini PV, Speckmann T, Lynn FC (2019) Friend and foe: β-cell Ca2+ signaling and the development of diabetes. Mol Metab 21:1–12. https://doi.org/10.1016/j.molmet.2018.12.007
Sano R (1833) Reed JC (2013) ER stress-induced cell death mechanisms. Biochim Biophys Acta 12:3460–3470. https://doi.org/10.1016/j.bbamcr.2013.06.028
Seino Y, Fukushima M, Yabe D (2010) GIP and GLP-1, the two incretin hormones: similarities and differences. J Diabestes Investig 1(1–2):8–23. https://doi.org/10.1111/j.2040-1124.2010.00022.x
Sha W, Hu F, Bu S (2020) Mitochondrial dysfunction and pancreatic islet β-cell failure. Exp Ther Med 20(6):266. https://doi.org/10.3892/etm.2020.9396
Shyng S, Nichols CG (1997) Octameric stoichiometry of the KATP chnnel complex. J Gen Physiol 110(6):655–664. https://doi.org/10.1085/jgp.110.6.655
Sneyd J, Tsaneva-Atanasova K, Reznikov V, Bai Y, Sanderson MJ, Yule DI (2006) A method for determining the dependence of calcium oscillations on inositol trisphosphate oscillations. Proc Natl Acad Sci U S A 103(6):1675–1680. https://doi.org/10.1073/pnas.0506135103
Stak K, Gotfredsen CF, Lundsgaard D, Hansen JB, Sturis J, Markholst H (2004) Improved beta-cell survival and reduced insulitis in a type 1 diabetic rat model after treatment with a beta-cell-selective K(ATP) channel opener. Diabetes 53(4):1089–1095. https://doi.org/10.2337/diabetes.53.4.1089
Sukumaran P, Schaar A, Sun Y, Singh BB (2016) Functional role of TRP channels in modulating ER stress and autophagy. Cell Calcium 60(2):123–132. https://doi.org/10.1016/j.ceca.2016.02.012
Sukumaran P, Nascimento Da Conceicao V, Sun Y, Ahamad N, Saraiva LR, Selvaraj S, Singh BB (2021) Calcium signaling regulates autophagy and apoptosis. Cells 10(8):2125. https://doi.org/10.3390/cells10082125
Tong X, Kono T, Anderson-Baucum EK, Yamamoto W, Gilon P, Lebeche D, Day RN, Shull GE, Evans-Molina C (2016) SERCA2 deficiency impairs pancreatic β-cell function in response to diet-induced obesity. Diabetes 65(10):3039–3052. https://doi.org/10.2337/db16-0084
Wali JA, Masters SL, Thomas HE (2013) Linking metabolic abnormalities to apoptotic pathways in beta cells in type 2 diabetes. Cells 2(2):266–283. https://doi.org/10.3390/cells2020266
Wang M, Wey S, Zhang Y, Ye R, Lee AS (2009) Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid Redox Signal 11(9):2307–2316. https://doi.org/10.1089/ars.2009.2485
Yamade T, Ishihara H, Tamura A, Takahashi R, Yamaguchi S, Takei D, Tokita A, Satake C, Tashiro F, Katagiri H, Aburatni H, Miyazaki J, Oka Y (2006) WFS1-deficiency increases endoplasmic reticulum stress, impairs cell cycle progression and triggers the apoptotic pathway specifically in pancreatic beta cells. Hum Mol Genet 15(10):1600–1609. https://doi.org/10.1093/hmg/ddl081
Yamamoto WR, Bone RN, Sohn P, Syed F, Reissaus CA, Mosley AL, Wijeratne AB, True JD, Tong X, Kono T, Evans-Molina C (2019) Endoplasmic reticulum stress alters ryanodine receptor function in the murine pancreatic β-cell. J Biol Chem 294(1):168–181. https://doi.org/10.1074/jbc,RA118.005683
Yang BC, Wu SY, Leung PS (2020) Alcohol ingestion induces pancreatic islet dysfunction and apoptosis via mediation of FGF21 resistance. Ann Transl Med 8(6):310. https://doi.org/10.21037/atm.2020.02.129
Yazbeck P, Tauseef M, Kruse K, Amin MR, Sheikh R, Feske S, Komarova Y, Mehta D (2017) STIM1 phosphorylation at Y361 recruits ORAI1 to STIM1 puncta and induces entry. Sci Rep 7:42758. https://doi.org/10.1038/srep42758
Yokoi N, Gheni G, Takahashi H, Seino S (2016) β-Cell glutanmate signaling: its role in incretin-induced insulin secretion. J Diabetes Investing 7(Suppl 1):38–43. https://doi.org/10.1111/jdi.12468
You S, Zheng J, Chen Y, Huang H (2022) Research progress on the mechanism of beta-cell apoptosis in type 2 diabetes mellitus. Front Endocrinol (Lausanne) 13:976465. https://doi.org/10.3389/fendo.2022.976465
Yu W, Niwa T, Miura Y, Horio F, Teradaira S, Ribar TJ, Means AR, Hasegawa Y, Senda T, Niki I (2002) Calmodulin overexpression cause Ca2+ -dependent apoptosis of pancreatic beta cells, which can be prevented by inhibition of nitric oxide synthase. Lab Invest 82(9):1229–1239. https://doi.org/10.1097/01.lab.0000027921.01548.c5
Yudin Y, Rohacs T (2012) Regulation of TRPM8 channel activity. Mol Cell Endocrinol 353(1–2):68–74. https://doi.org/10.1016/j.mce.2011.10.023
Zarayskiy V, Monje F, Peter K, Csutora P, Khodorov BI, Bolotina VM (2007) Store-operated Orai1 and IP3R-operated TRPC1 channel. Channels 1(2):246–252. https://doi.org/10.4161/chan.4835
Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD (2005) Stim1 is a ca sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 437(7060):902–905. https://doi.org/10.1038/nature04147
Zhang IX, Ren J, Vadrevu S, Raghavan M, Satin LS (2020) ER stress increases store-operated Ca2+ entry (SOCE) and augments basal insulin secretion in pancreatic beta cells. J Biol Chem 295(17):5685–5700. https://doi.org/10.1074/jbc.RA120.012721
Zheng D, Wang G, Li S, Fan GC (1852) Peng T (2015) Calpain-1 induces endoplasmic reticulum stress in promoting cardiomyocyte apoptosis following hypoxia/reoxygenation. Biochim Biophys Acta 5:882–892. https://doi.org/10.1016/j.bbadis.2015.01.019
Funding
This study was supported by grants from the National Research Foundation of Korea (NRF) Grant funded by the Korean Government (MSIP) (Grant No. 2021R1A2C2004171, 2021R1C1C2011264, 2021R1A2C1011233) and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health and Welfare, Republic of Korea (Grant No. HI14C1324).
Author information
Authors and Affiliations
Contributions
S.S.E. and I.S.S. organized and reviewed the manuscript. S.S.K. and J.H.Y. drew the figures. S.D.K. organized and wrote the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, SE., Shin, SK., Ju, H.Y. et al. Role of cytosolic and endoplasmic reticulum Ca2+ in pancreatic beta-cells: pros and cons. Pflugers Arch - Eur J Physiol 476, 151–161 (2024). https://doi.org/10.1007/s00424-023-02872-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-023-02872-2