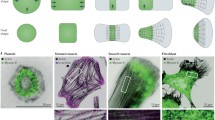
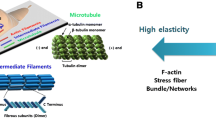
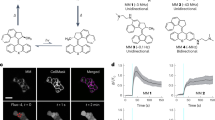
Abstract
Cell contraction plays an important role in many physiological and pathophysiological processes. This includes functions in skeletal, heart, and smooth muscle cells, which lead to highly coordinated contractions of multicellular assemblies, and functions in non-muscle cells, which are often highly localized in subcellular regions and transient in time. While the regulatory processes that control cell contraction in muscle cells are well understood, much less is known about cell contraction in non-muscle cells. In this review, we focus on the mechanisms that control cell contraction in space and time in non-muscle cells, and how they can be investigated by light-based methods. The review particularly focusses on signal networks and cytoskeletal components that together control subcellular contraction patterns to perform functions on the level of cells and tissues, such as directional migration and multicellular rearrangements during development. Key features of light-based methods that enable highly local and fast perturbations are highlighted, and how experimental strategies can capitalize on these features to uncover causal relationships in the complex signal networks that control cell contraction.





Similar content being viewed by others
Data availability
This declaration is not applicable.
References
Acharya BR, Wu SK, Lieu ZZ, Parton RG, Grill SW, Bershadsky AD, Gomez GA, Yap AS (2017) Mammalian diaphanous 1 mediates a pathway for e-cadherin to stabilize epithelial barriers through junctional contractility. Cell Rep 18:2854–2867. https://doi.org/10.1016/j.celrep.2017.02.078
Alvarado J, Sheinman M, Sharma A, Mackintosh FC, Koenderink GH (2013) Molecular motors robustly drive active gels to a critically connected state. Nat Phys 9(9):591–597. https://doi.org/10.1038/nphys2715
Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K (1996) Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem 271:20246–20249. https://doi.org/10.1074/jbc.271.34.20246
Arber S, Barbayannis FA, Hanser H, Schnelder C, Stanyon CA, Bernards O, Caroni P (1998) Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature 393(6687):805–809. https://doi.org/10.1038/31729
Arnold TR, Stephenson RE, Miller AL (2017) Rho GTPases and actomyosin: partners in regulating epithelial cell-cell junction structure and function. Exp Cell Res 358:20–30. https://doi.org/10.1016/J.YEXCR.2017.03.053
Ballister ER, Aonbangkhen C, Mayo AM, Lampson MA, Chenoweth DM (2014) Localized light-induced protein dimerization in living cells using a photocaged dimerizer. Nat Commun 5:5475. https://doi.org/10.1038/ncomms6475
Beach JR, Bruun KS, Shao L, Li D, Swider Z, Remmert K, Zhang Y, Conti MA, Adelstein RS, Rusan NM, Betzig E, Hammer JA (2017) Actin dynamics and competition for myosin monomer govern the sequential amplification of myosin filaments. Nat Cell Biol 19:85–93. https://doi.org/10.1038/NCB3463
Bement WM, Leda M, Moe AM, Kita AM, Larson ME, Golding AE, Pfeuti C, Su KC, Miller AL, Goryachev AB, von Dassow G (2015) Activator-inhibitor coupling between Rho signalling and actin assembly makes the cell cortex an excitable medium. Nat Cell Biol 17:1471–1483. https://doi.org/10.1038/ncb3251
Berlew EE, Kuznetsov IA, Yamada K, Bugaj LJ, Boerckel JD, Chow BY (2021) Single-component optogenetic tools for inducible RhoA GTPase signaling. Adv Biol 5:2100810. https://doi.org/10.1002/ADBI.202100810
Blanchard GB, Murugesu S, Adams RJ, Martinez-Arias A, Gorfinkiel N (2010) Cytoskeletal dynamics and supracellular organisation of cell shape fluctuations during dorsal closure. Development 137:2743–2752. https://doi.org/10.1242/dev.045872
Blaser H, Reichman-Fried M, Castanon I, Dumstrei K, Marlow FLL, Kawakami K, Solnica-Krezel L, Heisenberg CP, Raz E (2006) Migration of zebrafish primordial germ cells: a role for myosin contraction and cytoplasmic flow. Dev Cell 11:613–627. https://doi.org/10.1016/J.DEVCEL.2006.09.023
Bray D, Heath J, Moss D (1986) The membrane-associated ‘cortex’ of animal cells: its structure and mechanical properties. J Cell Sci 1986:71–88. https://doi.org/10.1242/JCS.1986.SUPPLEMENT_4.5
Burnette DT, Manley S, Sengupta P, Sougrat R, Davidson MW, Kachar B, Lippincott-Schwartz J (2011) A role for actin arcs in the leading-edge advance of migrating cells. Nat Cell Biol 13(4):371–382. https://doi.org/10.1038/ncb2205
Carramusa L, Ballestrem C, Zilberman Y, Bershadsky AD (2007) Mammalian diaphanous-related formin Dia1 controls the organization of E-cadherin-mediated cell-cell junctions. J Cell Sci 120:3870–3882. https://doi.org/10.1242/JCS.014365
Castella LF, Buscemi L, Godbout C, Meister JJ, Hinz B (2010) A new lock-step mechanism of matrix remodelling based on subcellular contractile events. J Cell Sci 123:1751–1760. https://doi.org/10.1242/JCS.066795
Chen M, Pan H, Sun L, Shi P, Zhang Y, Li L, Huang Y, Chen J, Jiang P, Fang X, Wu C, Chen Z (2020) Structure and regulation of human epithelial cell transforming 2 protein. Proc Natl Acad Sci U S A 117:1027–1035. https://doi.org/10.1073/PNAS.1913054117
Chen X, Venkatachalapathy M, Kamps D, Weigel S, Kumar R, Orlich M, Garrecht R, Hirtz M, Niemeyer CM, Wu YW, Dehmelt L (2017) “Molecular activity painting”: switch-like, light-controlled perturbations inside living cells. Angew Chem Int Ed Engl 56:5916–5920. https://doi.org/10.1002/anie.201611432
Chhabra ES, Higgs HN (2007) The many faces of actin: matching assembly factors with cellular structures. Nat Cell Biol 9(10):1110–1121. https://doi.org/10.1038/ncb1007-1110
Dasbiswas K, Hu S, Schnorrer F, Safran SA, Bershadsky AD (2018) Ordering of myosin II filaments driven by mechanical forces: experiments and theory. Phil Trans R Soc B Biol Sci 373. https://doi.org/10.1098/RSTB.2017.0114
David DJ, Tishkina A, Harris TJ (2010) The PAR complex regulates pulsed actomyosin contractions during amnioserosa apical constriction in Drosophila. Development 137:1645–1655. https://doi.org/10.1242/dev.044107
Desai R, Sarpal R, Ishiyama N, Pellikka M, Ikura M, Tepass U (2013) Monomeric α-catenin links cadherin to the actin cytoskeleton. Nat Cell Biol 15(3):261–273. https://doi.org/10.1038/ncb2685
Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S (2011) Role of YAP/TAZ in mechanotransduction. Nature 474(7350):179–183. https://doi.org/10.1038/nature10137
Fehon RG, McClatchey AI, Bretscher A (2010) Organizing the cell cortex: the role of ERM proteins. Nat Rev Mol Cell Biol 11(4):276–287. https://doi.org/10.1038/nrm2866
Fujiwara K, Pollard TD (1976) Fluorescent antibody localization of myosin in the cytoplasm, cleavage furrow, and mitotic spindle of human cells. J Cell Biol 71:848–875. https://doi.org/10.1083/JCB.71.3.848
Furst DO, Osborn M, Nave R, Weber K (1988) The organization of titin filaments in the half-sarcomere revealed by monoclonal antibodies in immunoelectron microscopy: a map of ten nonrepetitive epitopes starting at the Z line extends close to the M line. J Cell Biol 106:1563–1572. https://doi.org/10.1083/JCB.106.5.1563
Garcia-Mata R, Boulter E, Burridge K (2011) The “invisible hand”: regulation of RHO GTPases by RHOGDIs. Nat Rev Mol Cell Biol 12:493–504. https://doi.org/10.1038/nrm3153
Glaven JA, Whitehead IP, Nomanbhoy T, Kay R, Cerione RA (1996) Lfc and Lsc oncoproteins represent two new guanine nucleotide exchange factors for the Rho GTP-binding protein. J Biol Chem 271:27374–27381. https://doi.org/10.1074/JBC.271.44.27374
Gordon AM, Homsher E, Regnier M (2000) Regulation of contraction in striated muscle. Physiol Rev 80:853–924. https://doi.org/10.1152/PHYSREV.2000.80.2.853/ASSET/IMAGES/LARGE/9J0200077008.JPEG
Goryachev AB, Leda M, Miller AL, von Dassow G, Bement WM (2016) How to make a static cytokinetic furrow out of traveling excitable waves. Small GTPases 7:65–70. https://doi.org/10.1080/21541248.2016.1168505
Graessl M, Koch J, Calderon A, Kamps D, Banerjee S, Mazel T, Schulze N, Jungkurth JK, Patwardhan R, Solouk D, Hampe N, Hoffmann B, Dehmelt L, Nalbant P (2017) An excitable Rho GTPase signaling network generates dynamic subcellular contraction patterns. J Cell Biol 216:4271–4285. https://doi.org/10.1083/jcb.201706052
Guilluy C, Garcia-Mata R, Burridge K (2011) Rho protein crosstalk: another social network? Trends Cell Biol 21:718–726. https://doi.org/10.1016/j.tcb.2011.08.002
Guo H, Swan M, He B (2022) Optogenetic inhibition of actomyosin reveals mechanical bistability of the mesoderm epithelium during Drosophila mesoderm invagination. Elife 11. https://doi.org/10.7554/ELIFE.69082
Harburger DS, Calderwood DA (2009) Integrin signalling at a glance. J Cell Sci 122:159–163. https://doi.org/10.1242/JCS.018093
Hashimoto H, Munro E (2019) Differential expression of a classic cadherin directs tissue-level contractile asymmetry during neural tube closure. Dev Cell 51:158–172.e4. https://doi.org/10.1016/J.DEVCEL.2019.10.001
Hirokawa N, Keller TCS, Chasan R, Mooseker MS (1983) Mechanism of brush border contractility studied by the quick-freeze, deep-etch method. J Cell Biol 96:1325–1336. https://doi.org/10.1083/Jcb.96.5.1325
Hirokawa N, Tilnfy LG, Fujiwara K, Heuser JE (1982) Organization of actin, myosin, and intermediate the brush border of intestinal epithelial cells filaments in the brush border of intestinal epithelial cells. J Cell Biol 94(2):425–443. https://doi.org/10.1083/jcb.94.2.425
Hodge RG, Ridley AJ (2016) Regulating Rho GTPases and their regulators. Nat Rev Mol Cell Biol 17:496–510. https://doi.org/10.1038/nrm.2016.67
Hotulainen P, Lappalainen P (2006) Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J Cell Biol 173:383–394. https://doi.org/10.1083/JCB.200511093/VIDEO-10
Huxley HE (2004) Fifty years of muscle and the sliding filament hypothesis. Eur J Biochem 271:1403–1415. https://doi.org/10.1111/J.1432-1033.2004.04044.X
Hynes RO (2002) Integrins: bidirectional, allosteric signaling machines. Cell 110:673–687. https://doi.org/10.1016/S0092-8674(02)00971-6
Izquierdo E, Quinkler T, De Renzis S (2018) Guided morphogenesis through optogenetic activation of Rho signalling during early Drosophila embryogenesis. Nat Commun 9(1):1–13. https://doi.org/10.1038/s41467-018-04754-z
Kamps D, Dehmelt L (2017) Deblurring signal network dynamics. ACS Chem Biol 12:2231–2239. https://doi.org/10.1021/ACSCHEMBIO.7B00451
Kamps D, Koch J, Juma VO, Campillo-Funollet E, Graessl M, Banerjee S, Mazel T, Chen X, Wu YW, Portet S, Madzvamuse A, Nalbant P, Dehmelt L (2020) Optogenetic tuning reveals Rho amplification-dependent dynamics of a cell contraction signal network. Cell Rep 33:108467. https://doi.org/10.1016/j.celrep.2020.108467
Kanchanawong P, Shtengel G, Pasapera AM, Ramko EB, Davidson MW, Hess HF, Waterman CM (2010) Nanoscale architecture of integrin-based cell adhesions. Nature 468(7323):580–584. https://doi.org/10.1038/nature09621
Kennedy MJ, Hughes RM, Peteya LA, Schwartz JW, Ehlers MD, Tucker CL (2010) Rapid blue-light-mediated induction of protein interactions in living cells. Nat Methods 7:973–975. https://doi.org/10.1038/nmeth.1524
Kimura K, Fukata Y, Matsuoka Y, Bennett V, Matsuura Y, Okawa K, Iwamatsu A, Kaibuchi K (1998) Regulation of the association of adducin with actin filaments by Rho- associated kinase (Rho-kinase) and myosin phosphatase. J Biol Chem 273:5542–5548. https://doi.org/10.1074/jbc.273.10.5542
Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A (1979) Kaibuchi K (1996) Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273:245–248. https://doi.org/10.1126/SCIENCE.273.5272.245
Klughammer N, Bischof J, Schnellbächer ND, Callegari A, Lénárt P, Schwarz US (2018) Cytoplasmic flows in starfish oocytes are fully determined by cortical contractions. PLoS Comput Biol 14:e1006588. https://doi.org/10.1371/JOURNAL.PCBI.1006588
Koenderink GH, Paluch EK (2018) Architecture shapes contractility in actomyosin networks. Curr Opin Cell Biol 50:79–85. https://doi.org/10.1016/J.CEB.2018.01.015
Kolodney MS, Thimgan MS, Honda HM, Tsai G, Yee HF (1999) Ca2+-independent myosin II phosphorylation and contraction in chicken embryo fibroblasts. J Physiol 515:87–92. https://doi.org/10.1111/J.1469-7793.1999.087AD.X
Kowalczyk M, Kamps D, Wu Y, Dehmelt L, Nalbant P (2021) Monitoring the response of multiple signal network components to acute chemo-optogenetic perturbations in living cells. Chembiochem e202100582. https://doi.org/10.1002/cbic.202100582
Le Bras S, Le Borgne R (2014) Epithelial cell division - multiplying without losing touch. J Cell Sci 127:5127–5137. https://doi.org/10.1242/JCS.151472/259089/AM/EPITHELIAL-CELL-DIVISION-MULTIPLYING-WITHOUT
Lee CS, Choi CK, Shin EY, Schwartz MA, Kim EG (2010) Myosin II directly binds and inhibits Dbl family guanine nucleotide exchange factors: a possible link to Rho family GTPases. J Cell Biol 190:663–674. https://doi.org/10.1083/jcb.201003057
Lee S, Kumar S (2020) Cofilin is required for polarization of tension in stress fiber networks during migration. J Cell Sci 133. https://doi.org/10.1242/JCS.243873/VIDEO-10
Lee S, Park H, Kyung T, Kim NY, Kim S, Kim J, Do HW (2014) Reversible protein inactivation by optogenetic trapping in cells. Nat Methods 11:633–636. https://doi.org/10.1038/NMETH.2940
Legate KR, Wickström SA, Fässler R (2009) Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev 23:397–418. https://doi.org/10.1101/GAD.1758709
Lehtimaki J, Hakala M, Lappalainen P (2017) Actin filament structures in migrating cells. Handb Exp Pharmacol 235:123–152. https://doi.org/10.1007/164_2016_28/FIGURES/2
Lehtimäki JI, Rajakylä EK, Tojkander S, Lappalainen P (2021) Generation of stress fibers through myosin-driven reorganization of the actin cortex. Elife 10:1–43. https://doi.org/10.7554/ELIFE.60710
Leung DW, Otomo C, Chory J, Rosen MK (2008) Genetically encoded photoswitching of actin assembly through the Cdc42-WASP-Arp2/3 complex pathway. Proc Natl Acad Sci U S A 105:12797–12802. https://doi.org/10.1073/PNAS.0801232105/SUPPL_FILE/0801232105SI.PDF
Leung T, Chen X-Q, Manser E, Lim L (2023) The p160 RhoA-binding kinase ROKα is a member of a kinase family and is involved in the reorganization of the cytoskeleton 16:5313–5327. https://doi.org/10.1128/MCB.16.10.5313
Levskaya A, Weiner OD, Lim WA, Voigt CA (2009) Spatiotemporal control of cell signalling using a light-switchable protein interaction. Nature 461:997–1001. https://doi.org/10.1038/nature08446
Litschko C, Brühmann S, Csiszár A, Stephan T, Dimchev V, Damiano-Guercio J, Junemann A, Körber S, Winterhoff M, Nordholz B, Ramalingam N, Peckham M, Rottner K, Merkel R, Faix J (2019) Functional integrity of the contractile actin cortex is safeguarded by multiple Diaphanous-related formins. Proc Natl Acad Sci U S A 116:3594–3603. https://doi.org/10.1073/PNAS.1821638116/SUPPL_FILE/PNAS.1821638116.SM10.AVI
Luo W, Yu C-h, Lieu ZZ, Allard J, Mogilner A, Sheetz MP, Bershadsky AD (2013) Analysis of the local organization and dynamics of cellular actin networks. J Cell Biol 202:1057–1073. https://doi.org/10.1083/JCB.201210123/VIDEO-8
Maddox AS, Burridge K (2003) RhoA is required for cortical retraction and rigidity during mitotic cell rounding. J Cell Biol 160:255–265. https://doi.org/10.1083/jcb.200207130
Maitre JL, Niwayama R, Turlier H, Nedelec F, Hiiragi T (2015) Pulsatile cell-autonomous contractility drives compaction in the mouse embryo. Nat Cell Biol 17:849–855. https://doi.org/10.1038/ncb3185
Martin AC, Gelbart M, Fernandez-Gonzalez R, Kaschube M, Wieschaus EF (2010) Integration of contractile forces during tissue invagination. J Cell Biol 188:735–749. https://doi.org/10.1083/jcb.200910099
Martin AC, Kaschube M, Wieschaus EF (2009) Pulsed contractions of an actin-myosin network drive apical constriction. Nature 457:495–499. https://doi.org/10.1038/nature07522
Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K (1996) Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J 15:2208–2216. https://doi.org/10.1002/J.1460-2075.1996.TB00574.X
Matsui T, Maeda M, Doi Y, Yonemura S, Amano M, Kaibuchi K, Tsukita S, Tsukita S (1998) Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J Cell Biol 140:647–657. https://doi.org/10.1083/JCB.140.3.647
McGregor A, Blanchard AD, Rowe AJ, Critchley DR (1994) Identification of the vinculin-binding site in the cytoskeletal protein α-actinin. Biochem J 301:225–233. https://doi.org/10.1042/BJ3010225
Medina F, Carter AM, Dada O, Gutowski S, Hadas J, Chen Z, Sternweis PC (2013) Activated RhoA is a positive feedback regulator of the Lbc family of Rho guanine nucleotide exchange factor proteins. J Biol Chem 288:11325–11333. https://doi.org/10.1074/jbc.M113.450056
Meinhardt H (2004) Out-of-phase oscillations and traveling waves with unusual properties: the use of three-component systems in biology. Physica D 199:264–277
Méry A, Ruppel A, Revilloud J, Balland M, Cappello G, Boudou T (2023) Light-driven biological actuators to probe the rheology of 3D microtissues. Nat Commun 14(1):1–12. https://doi.org/10.1038/s41467-023-36371-w
Michaud A, Leda M, Swider ZT, Kim S, He J, Landino J, Valley JR, Huisken J, Goryachev AB, Von Dassow G, Bement WM (2022) A versatile cortical pattern-forming circuit based on Rho, F-actin, Ect2, and RGA-3/4. J Cell Biol 221. https://doi.org/10.1083/JCB.202203017
Michaud A, Swider ZT, Landino J, Leda M, Miller AL, von Dassow G, Goryachev AB, Bement WM (2021) Cortical excitability and cell division. Curr Biol 31:R553–R559. https://doi.org/10.1016/j.cub.2021.02.053
Munjal A, Philippe JM, Munro E, Lecuit T (2015) A self-organized biomechanical network drives shape changes during tissue morphogenesis. Nature 524:351–355. https://doi.org/10.1038/nature14603
Nalbant P, Dehmelt L (2018) Exploratory cell dynamics: a sense of touch for cells? Biol Chem 399:809–819. https://doi.org/10.1515/hsz-2017-0341
Nishikawa M, Naganathan SR, Julicher F, Grill SW (2017) Controlling contractile instabilities in the actomyosin cortex. Elife 6. https://doi.org/10.7554/eLife.19595
Nobes CD, Hall A (1995) Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81:53–62
Oda H, Tsukita S (2001) Real-time imaging of cell-cell adherens junctions reveals that Drosophila mesoderm invagination begins with two phases of apical constriction of cells. J Cell Sci 114:493–501. https://doi.org/10.1242/JCS.114.3.493
Ohashi K, Nagata K, Maekawa M, Ishizaki T, Narumiya S, Mizuno K (2000) Rho-associated kinase ROCK activates LIM-kinase 1 by phosphorylation at threonine 508 within the activation loop. J Biol Chem 275:3577–3582. https://doi.org/10.1074/jbc.275.5.3577
Pollard TD, O’Shaughnessy B (2019) Molecular mechanism of cytokinesis. https://doi.org/10.1146/annurev-biochem-062917-012530 88:661–689. https://doi.org/10.1146/ANNUREV-BIOCHEM-062917-012530
Qin X, Hannezo E, Mangeat T, Liu C, Majumder P, Liu J, Choesmel-Cadamuro V, McDonald JA, Liu Y, Yi B, Wang X (2018) A biochemical network controlling basal myosin oscillation. Nat Commun 9(1):1–15. https://doi.org/10.1038/s41467-018-03574-5
Ridley AJ (2011) Life at the leading edge. Cell 145:1012–1022. https://doi.org/10.1016/J.CELL.2011.06.010
Ridley AJ, Hall A (1992) The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70:389–399. https://doi.org/10.1016/0092-8674(92)90163-7
Rolo A, Skoglund P, Keller R (2009) Morphogenetic movements driving neural tube closure in Xenopus require myosin IIB. Dev Biol 327:327–338. https://doi.org/10.1016/J.YDBIO.2008.12.009
Sahai E, Marshall CJ (2003) Differing modes for tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol 5:711–719. https://doi.org/10.1038/NCB1019
Sampayo RG, Sakamoto M, Wang M, Kumar S, Schaffer DV (2023) Mechanosensitive stem cell fate choice is instructed by dynamic fluctuations in activation of Rho GTPases. Proc Natl Acad Sci 120:e2219854120. https://doi.org/10.1073/PNAS.2219854120
Sawyer JM, Harrell JR, Shemer G, Sullivan-Brown J, Roh-Johnson M, Goldstein B (2010) Apical constriction: a cell shape change that can drive morphogenesis. Dev Biol 341:5–19. https://doi.org/10.1016/J.YDBIO.2009.09.009
Schroeder TE (1973) Actin in dividing cells: contractile ring filaments bind heavy meromyosin. Proc Natl Acad Sci 70:1688–1692. https://doi.org/10.1073/PNAS.70.6.1688
Schulze N, Graessl M, Blancke Soares A, Geyer M, Dehmelt L, Nalbant P (2014) FHOD1 regulates stress fiber organization by controlling the dynamics of transverse arcs and dorsal fibers. J Cell Sci 127:1379–1393. https://doi.org/10.1242/jcs.134627
Sluysmans S, Vasileva E, Spadaro D, Shah J, Rouaud F, Citi S (2017) The role of apical cell–cell junctions and associated cytoskeleton in mechanotransduction. Biol Cell 109:139–161. https://doi.org/10.1111/BOC.201600075
Smutny M, Cox HL, Leerberg JM, Kovacs EM, Conti MA, Ferguson C, Hamilton NA, Parton RG, Adelstein RS, Yap AS (2010) Myosin II isoforms identify distinct functional modules that support integrity of the epithelial zonula adherens. Nat Cell Biol 12(7):696–702. https://doi.org/10.1038/ncb2072
Strickland D, Lin Y, Wagner E, Hope CM, Zayner J, Antoniou C, Sosnick TR, Weiss EL, Glotzer M (2012) TULIPs: tunable, light-controlled interacting protein tags for cell biology. Nat Methods 9:379–384. https://doi.org/10.1038/nmeth.1904
Sumi A, Hayes P, D’Angelo A, Colombelli J, Salbreux G, Dierkes K, Solon J (2018) Adherens junction length during tissue contraction is controlled by the mechanosensitive activity of actomyosin and junctional recycling. Dev Cell 47:453-463.e3. https://doi.org/10.1016/j.devcel.2018.10.025
Svitkina TM (2020) Actin cell cortex: structure and molecular organization. Trends Cell Biol 30:556–565. https://doi.org/10.1016/j.tcb.2020.03.005
Svitkina TM, Verkhovsky AB, McQuade KM, Borisy GG (1997) Analysis of the actin-myosin II system in fish epidermal keratocytes: mechanism of cell body translocation. J Cell Biol 139:397–415
Sweeney HL, Hammers DW (2018) Muscle contraction. Cold Spring Harb Perspect Biol 10. https://doi.org/10.1101/CSHPERSPECT.A023200
Swider ZT, Michaud A, Leda M, Landino J, Goryachev AB, Bement WM (2022) Cell cycle and developmental control of cortical excitability in Xenopus laevis. Mol Biol Cell 33. https://doi.org/10.1091/MBC.E22-01-0025
Takeya R, Taniguchi K, Narumiya S, Sumimoto H (2008) The mammalian formin FHOD1 is activated through phosphorylation by ROCK and mediates thrombin-induced stress fibre formation in endothelial cells. EMBO J 27:618–628. https://doi.org/10.1038/EMBOJ.2008.7
Tatsumoto T, Xie X, Blumenthal R, Okamoto I, Miki T (1999) Human ECT2 is an exchange factor for Rho GTPases, phosphorylated in G2/M phases, and involved in cytokinesis. J Cell Biol 147:921–927. https://doi.org/10.1083/JCB.147.5.921
Taubenberger AV, Baum B, Matthews HK (2020) The mechanics of mitotic cell rounding. Front Cell Dev Biol 8:687. https://doi.org/10.3389/FCELL.2020.00687/BIBTEX
Thiery JP, Sleeman JP (2006) Complex networks orchestrate epithelial–mesenchymal transitions. Nat Rev Mol Cell Biol 7(2):131–142. https://doi.org/10.1038/nrm1835
Tojkander S, Gateva G, Lappalainen P (2012) Actin stress fibers–assembly, dynamics and biological roles. J Cell Sci 125:1855–1864. https://doi.org/10.1242/jcs.098087
Turing AM (1952) The chemical basis of morphogenesis. Philos Trans R Soc Lond B Biol Sci 237:37–72
Tyson JJ, Chen KC, Novak B (2003) Sniffers, buzzers, toggles and blinkers: dynamics of regulatory and signaling pathways in the cell. Curr Opin Cell Biol 15:221–231
Valencia FR, Sandoval E, Du J, Iu E, Liu J, Plotnikov SV (2021) Force-dependent activation of actin elongation factor mDia1 protects the cytoskeleton from mechanical damage and promotes stress fiber repair. Dev Cell 56:3288-3302.e5. https://doi.org/10.1016/j.devcel.2021.11.004
Valon L, Marín-Llauradó A, Wyatt T, Charras G, Trepat X (2017) Optogenetic control of cellular forces and mechanotransduction. Nat Commun 8. https://doi.org/10.1038/NCOMMS14396
van den Boom F, Dussmann H, Uhlenbrock K, Abouhamed M, Bahler M (2007) The myosin IXb motor activity targets the myosin IXb RhoGAP domain as cargo to sites of actin polymerization. Mol Biol Cell 18:1507–1518. https://doi.org/10.1091/mbc.E06-08-0771
Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR (2009) Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol 10:778–790. https://doi.org/10.1038/nrm2786
Wang H, Vilela M, Winkler A, Tarnawski M, Schlichting I, Yumerefendi H, Kuhlman B, Liu R, Danuser G, Hahn KM (2016) LOVTRAP: an optogenetic system for photoinduced protein dissociation. Nat Methods 13:755–758. https://doi.org/10.1038/nmeth.3926
Wang Z, Raunser S (2023) Structural biochemistry of muscle contraction. Annu Rev Biochem 92. https://doi.org/10.1146/ANNUREV-BIOCHEM-052521-042909
Watanabe N, Kato T, Fujita A, Ishizaki T, Narumiya S (1999) Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat Cell Biol 1:136–143. https://doi.org/10.1038/11056
Watanabe N, Madaule P, Reid T, Ishizaki T, Watanabe G, Kakizuka A, Saito Y, Nakao K, Jockusch BM, Narumiya S (1997) p140mDia, a mammalian homolog of Drosophila diaphanous, is a target protein for Rho small GTPase and is a ligand for profilin. EMBO J 16:3044–3056. https://doi.org/10.1093/EMBOJ/16.11.3044
Weiss A, Leinwand LA (2003) The mammalian myosin heavy chain gene family. https://doi.org/10.1146/annurev.cellbio121417 12:417–439. https://doi.org/10.1146/ANNUREV.CELLBIO.12.1.417
Weng M, Wieschaus E (2016) Myosin-dependent remodeling of adherens junctions protects junctions from Snail-dependent disassembly. J Cell Biol 212:219–229. https://doi.org/10.1083/JCB.201508056/VIDEO-3
Wennerberg K, Rossman KL, Der CJ (2005) The Ras superfamily at a glance. J Cell Sci 118:843–846. https://doi.org/10.1242/JCS.01660
Wu YI, Frey D, Lungu OI, Jaehrig A, Schlichting I, Kuhlman B, Hahn KM (2009) A genetically encoded photoactivatable Rac controls the motility of living cells. Nature 461:104–108. nature08241 [pii] https://doi.org/10.1038/nature08241
Yamamoto K, Miura H, Ishida M, Mii Y, Kinoshita N, Takada S, Ueno N, Sawai S, Kondo Y, Aoki K (2021) Optogenetic relaxation of actomyosin contractility uncovers mechanistic roles of cortical tension during cytokinesis. Nat Commun 12(1):1–13. https://doi.org/10.1038/s41467-021-27458-3
Zhou XX, Chung HK, Lam AJ (1979) Lin MZ (2012) Optical control of protein activity by fluorescent protein domains. Science 338:810–814. https://doi.org/10.1126/science.1226854
Funding
This work was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) Priority Program SPP 1926 under the grant number NA 413/4–1 to P. N. and J. W., and the DFG Principal Investigator grant DE 823/10–1 to L. D.
Author information
Authors and Affiliations
Contributions
P. N. and L. D. wrote the main manuscript text. All authors prepared the figures. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
This declaration is not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the special issue on Next-generation optogenetics in Pflügers Archiv—European Journal of Physiology.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nalbant, P., Wagner, J. & Dehmelt, L. Direct investigation of cell contraction signal networks by light-based perturbation methods. Pflugers Arch - Eur J Physiol 475, 1439–1452 (2023). https://doi.org/10.1007/s00424-023-02864-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-023-02864-2