Abstract
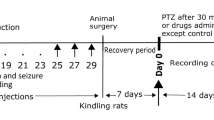
This study endeavoured to assess the effect of hemopressin (Hp), a nano peptide obtained from the alpha chain of hemoglobin, on chronic epileptic activity and its potential correlation with cannabinoid receptor type 1 (CB1). Male Wistar albino rats (230–260 g) were used. The kindling process was conducted by administering a sub-convulsant dose of pentylenetetrazol (PTZ) (35 mg/kg, i.p) three times a week for a maximum of 10 weeks. Tripolar electrodes and external cannula guides for intracerebroventricular (i.c.v) injections were surgically implanted in the skulls of kindled rats. On the day of the experiment, doses of Hp, AM-251, and ACEA were administered prior to the PTZ injections. Electroencephalography recordings and behavioural observations were conducted simultaneously for 30 min after the PTZ injection. The administration of Hp (0.6 μg, i.c.v) resulted in a decrease in epileptic activity. The CB1 receptor agonist ACEA (7.5 μg, i.c.v) showed an anticonvulsant effect, but the CB1 receptor antagonist AM-251 (0.5 μg, i.c.v) displayed a proconvulsant effect. The co-administration of Hp (0.6 μg, i.c.v) and ACEA (7.5 μg, i.c.v) and of Hp (0.6 μg, i.c.v) and AM-251 (0.5 μg, i.c.v) produced an anticonvulsant effect. However, when AM-251 was administered prior to Hp, it produced a proconvulsant impact that overrode Hp’s intended anticonvulsant effect. Interestingly, the co-administration of Hp (0.03 μg) + AM-251 (0.125 μg) unexpectedly exhibited an anticonvulsant effect. Electrophysiological and behavioural evaluations demonstrated the anticonvulsant effect of Hp in the present model, highlighting the possibility that Hp may act as an agonist for the CB1 receptor.







Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Agar E (2015) The role of cannabinoids and leptin in neurological diseases. Acta Neurol Scand 132(6):371–380
Al-Gailani L et al (2022) THE effect of general anesthetics on genetic absence epilepsy in WAG/Rij rats. Neurol Res 44(11):995–1005
Arslan G et al (2017) Interaction between urethane and cannabinoid CB1 receptor agonist and antagonist in penicillin-induced epileptiform activity. Acta Neurobiol Exp (Wars) 77(2):128–136
Aygun H et al (2020) Hemopressin increases penicillin-induced epileptiform activity in rats. Bratisl Lek Listy 121(1):37–42
Bahremand A et al (2009) Involvement of nitrergic system in the anticonvulsant effect of the cannabinoid CB(1) agonist ACEA in the pentylenetetrazole-induced seizure in mice. Epilepsy Res 84(2–3):110–119
Billakota S, Devinsky O, Marsh E (2019) Cannabinoid therapy in epilepsy. Curr Opin Neurol 32(2):220–226
Blair RE et al (2006) Activation of the cannabinoid type-1 receptor mediates the anticonvulsant properties of cannabinoids in the hippocampal neuronal culture models of acquired epilepsy and status epilepticus. J Pharmacol Exp Ther 317(3):1072–1078
Chen K et al (2007) Prevention of plasticity of endocannabinoid signaling inhibits persistent limbic hyperexcitability caused by developmental seizures. J Neurosci 27(1):46–58
Cremer CM et al (2009) Pentylenetetrazole-induced seizures affect binding site densities for GABA, glutamate and adenosine receptors in the rat brain. Neuroscience 163(1):490–499
Deshpande LS et al (2007) Endocannabinoids block status epilepticus in cultured hippocampal neurons. Eur J Pharmacol 558(1–3):52–59
Dodd GT et al (2010) The peptide hemopressin acts through CB1 cannabinoid receptors to reduce food intake in rats and mice. J Neurosci 30(21):7369–7376
Dodd GT et al (2013) Central functional response to the novel peptide cannabinoid, hemopressin. Neuropharmacology 71:27–36
Echegoyen J et al (2009) Single application of a CB1 receptor antagonist rapidly following head injury prevents long-term hyperexcitability in a rat model. Epilepsy Res 85(1):123–127
Ekonomou A, Smith AL, Angelatou F (2001) Changes in AMPA receptor binding and subunit messenger RNA expression in hippocampus and cortex in the pentylenetetrazole-induced “kindling” model of epilepsy. Brain Res Mol Brain Res 95(1–2):27–35
Emendato A et al (2018) Disordered Peptides Looking for Their Native Environment: Structural Basis of CB1 Endocannabinoid Receptor Binding to Pepcans. Front Mol Biosci 5:100
Fischer W, Kittner H (1998) Influence of ethanol on the pentylenetetrazol-induced kindling in rats. J Neural Transm (Vienna) 105(10–12):1129–1142
Freund TF, Katona I, Piomelli D (2003) Role of endogenous cannabinoids in synaptic signaling. Physiol Rev 83(3):1017–1066
Gholizadeh S et al (2007) Ultra-low dose cannabinoid antagonist AM251 enhances cannabinoid anticonvulsant effects in the pentylenetetrazole-induced seizure in mice. Neuropharmacology 53(6):763–770
Hama A, Sagen J (2011) Centrally mediated antinociceptive effects of cannabinoid receptor ligands in rat models of nociception. Pharmacol Biochem Behav 100(2):340–346
Heimann AS et al (2007) Hemopressin is an inverse agonist of CB1 cannabinoid receptors. Proc Natl Acad Sci U S A 104(51):20588–20593
Heimann AS et al (2021) Hemopressin as a breakthrough for the cannabinoid field. Neuropharmacology 183:108406
Hoffman AF, Lupica CR (2000) Mechanisms of cannabinoid inhibition of GABA(A) synaptic transmission in the hippocampus. J Neurosci 20(7):2470–2479
Karr L, Pan YZ, Rutecki PA (2010) CB1 receptor antagonism impairs the induction of epileptiform activity by group I metabotropic glutamate receptor activation. Epilepsia 51 Suppl 3(Suppl 3):121–5
Katona I et al (2006) Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci 26(21):5628–5637
Katona I, Freund TF (2008) Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat Med 14(9):923–930
Kozan R, Ayyildiz M, Agar E (2009) The effects of intracerebroventricular AM-251, a CB1-receptor antagonist, and ACEA, a CB1-receptor agonist, on penicillin-induced epileptiform activity in rats. Epilepsia 50(7):1760–1767
Pan JX et al (2014) Analgesic tolerance and cross-tolerance to the cannabinoid receptors ligands hemopressin, VD-hemopressin(α) and WIN55,212–2 at the supraspinal level in mice. Neurosci Lett 578:187–191
Pertwee RG (2010) Receptors and channels targeted by synthetic cannabinoid receptor agonists and antagonists. Curr Med Chem 17(14):1360–1381
Remigio GJ et al (2017) Corneal kindled C57BL/6 mice exhibit saturated dentate gyrus long-term potentiation and associated memory deficits in the absence of overt neuron loss. Neurobiol Dis 105:221–234
Rocha L, Ackermann RF, Engel J Jr (1996) Chronic and single administration of pentylenetetrazol modifies benzodiazepine receptor-binding: an autoradiographic study. Epilepsy Res 24(2):65–72
Scrima M et al (2010) Binding of the hemopressin peptide to the cannabinoid CB1 receptor: structural insights. Biochemistry 49(49):10449–10457
Sieradzan KA et al (2001) Cannabinoids reduce levodopa-induced dyskinesia in Parkinson’s disease: a pilot study. Neurology 57(11):2108–2111
van Rijn CM et al (2011) Endocannabinoid system protects against cryptogenic seizures. Pharmacol Rep 63(1):165–168
Wallace MJ, Martin BR, DeLorenzo RJ (2002) Evidence for a physiological role of endocannabinoids in the modulation of seizure threshold and severity. Eur J Pharmacol 452(3):295–301
Funding
This work was supported by The Scientific and Technological Research Council of Turkey (TUBITAK) [project number 215S808]. The funding source had no involvement in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
AA., LA., HA, and AH: Conducted the experiments. YK, SI, and MA: Analyzed the data. EA and AA: Wrote the manuscript. EA: Planned and supervised all experiments.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose other than the funding source (The Scientific and Technological Research Council of Turkey (TUBITAK) [project number 215S808]) mentioned in the section above.
Ethics approval
The experimental procedures were conducted following the European Union Directive (2010/63/EU) and the Turkish Legislations Acts of experimental animals. The study protocol was approved by the Local Ethical Committee of Ondokuz Mayis University (2015/55).
Consent to participate
Not applicable.
Patient consent
Not applicable.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Al-Kaleel, A., Aygun, H., Al-Gailani, L. et al. The electrophysiological and behavioral evaluation of the peptide hemopressin and cannabinoid CB1 receptor agonist and antagonist in pentylenetetrazol model of epilepsy in rats. Pflugers Arch - Eur J Physiol 475, 719–730 (2023). https://doi.org/10.1007/s00424-023-02814-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-023-02814-y