Abstract
Global warming and connected acidification of the world ocean attract a substantial amount of research efforts, in particular in a context of their impact on behaviour and metabolism of marine organisms, such as Cnidaria. Nevertheless, mechanisms underlying Cnidarians’ neural signalling and behaviour and their (possible) alterations due to the world ocean acidification remain poorly understood. Here we researched for the first time modulation of GABAA receptors (GABAARs) in Actinia equina (Cnidaria: Anthozoa) by pH fluctuations within a range predicted by the world ocean acidification scenarios for the next 80–100 years and by selective pharmacological activation. We found that in line with earlier studies on vertebrates, both changes of pH and activation of GABAARs with a selective allosteric agonist (diazepam) modulate electrical charge transfer through GABAAR and the whole-cell excitability. On top of that, diazepam modifies the animal behavioural reaction on startle response. However, despite behavioural reactions displayed by living animals are controlled by GABAARs, changes of pH do not alter them significantly. Possible mechanisms underlying the species resistance to acidification impact are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The present rise of atmospheric CO2 significantly increases the partial pressure of CO2 in the world oceans and, consequently, leads to ocean acidification (OA). Different model scenarios predict a decline in pH values of oceanic waters by up to 0.45 units by the year 2100 [7, 39], with profound consequences to marine ecosystems [14]. In this context, the potential impact of OA on coral cnidarians (Cnidaria: Anthozoa, Scleractinia) attracts understandably significant research efforts given the global role of coral reefs and their sensitivity to water pH and connected fluctuations of carbonate–bicarbonate balance. However, how the OA-driven responses alter the behaviour of the soft-bodied forms of anthozoans and modify their long-term ecological perspectives is to a large extent unknown. During the last decade, several research groups have reported a negligible (or even positive) impact of OA on the metabolism of sea anemones of species supporting microalgal endosymbionts of Symbiodinium genus due to rapid changes in endosymbionts’ biochemistry and photosynthesis capacity [16, 20, 40]. Nevertheless, the impact of OA on non-symbiotic sea anemones has yet to be determined.
From the neurophysiological perspective, sea anemones such as Actinia equina (A. equina) (Cnidaria: Anthozoa, Actiniaria) are “…for all intents and purposes, little more than guts with tentacles” [19]. This definition clearly reflects a limited set of reactions generated by a nervous system of the simplest morphology (nerve net) amongst metazoans [12]. Thus, the factors that could modulate the prominence and features of species’ behavioural profile within such a simple nervous system are of considerable interest. This area has a relatively recent and irregular research history, where the startle response of the non-symbiotic species A. equina is used as a quantifying gauge of anemones’ adaptability to climate change and the expression of their personality [5, 29].
GABAA-receptors (GABAARs) are the major inhibitory neurotransmitter receptors in the animal nervous system. These receptors and elements of the synthesis and degradation of their endogenous ligand γ-aminobutyric acid (GABA) have been repeatedly shown to be present in different actinia species [1, 10], whereas the functional signature of GABAARs was demonstrated in Cnidaria of other classes [17, 32]. The effects of GABAARs in vertebrates’ neural cells were demonstrated to be pH-dependent due to protonation of the receptors’ extracellular domain. Fluctuations of pH by a few tenths of a unit make a significant impact on neural cell excitability and inter-neuronal signalling [8, 21, 43]. The level of sensitivity to pH fluctuations depends on the GABAAR subunit composition [21]. In anthozoans GABAARs are likely to be of a different subunit composition to that in vertebrates’ cells. Therefore, anthozoans are a prospective object for research of pH-related functionality of GABAARs and of the impact of environmental pH fluctuations on neural signalling.
The GABAAR is a traditional target for the treatment of neural disorders, where the effects of GABAAR ligands of the benzodiazepine type (such as diazepam [DZP]) are commonly associated with corrections of individual behaviour and personality profile [24, 41, 42]. However, to the best of our knowledge, to date the functional effects of benzodiazepines in Anthozoa remain unknown.
Hence, in this study, we aimed to test the impact of pH fluctuations within the OA-related interval on the nervous system of A. equina at different levels: from a single GABAAR to the generation of the startle response by living animals, connecting these points through the functional profile of a separate excitable cell. Additionally, we intended to clarify the DZP impact on anthozoan GABAAR functions at different organizational levels (single receptor, single cell, and animal behaviour). Inasmuch sensitivity to environmental factors and bioactive compounds in invertebrates may differ between ecologically and geographically disengaged populations of the same species [26], we aimed to perform experiments on animals representing two populations separated both ecologically and geographically: A. equina from both the inter-tidal zone of the Scottish North Sea coast and from the Black Sea wherein tides are absent [2].
The inter-annual fluctuations of pH both in the North Sea and in the Black Sea are within a range of 7.5–8.5 [4, 35]. Therefore, to explore a potential impact of OA (acidification shift by ≤ 0.45 unit [7, 39]) on GABAAR-mediated effects and to compare these effects to those observed at pH characteristic to present conditions, we set out to perform experiments at a pH range from 8.5 to 7.0.
Materials and methods
Ethical statement
A. equina is not protected under either UK Animals (Scientific Procedures) Act 1986 nor listed in the general provisions of the European Directive (2010) on the protection of animals used for scientific purposes. Nevertheless, the study was conducted in accord with the ASAB Guidelines for the treatment of animals in teaching and research [6]. All animals involved into behavioural tests were then released near the point of collection. Environmental parameters in research aquariums were consistently within normal tolerance ranges for corresponding population.
Behavioural tests
Sea anemones of the North Sea population were collected in a tidal zone of a rocky coast to the north of the town of Dunbar (56° 0′ 11″ N, 2° 31′ 48″ W). Sea anemones of the Black Sea population were collected at a depth of 2–5 m near the shore of Zmiinyi Island (45° 15′ 36″ N, 30° 1′ 12″ E) [37]. A distance of 2–3 m was left between the animals collected, to avoid collecting cloned individuals [11]. Animals were housed in experimental aquaria of 1 m3 volume, half-filled (0.5 m3) with sea water. Water temperature was held at a level similar to that observed at the time of collection: 12 ± 1 °C for the North Sea animals and 27 ± 1 °C for the Black Sea animals, bubbled with air compressors. Evaporating sea water volumes were replaced with distilled water. Animals were kept at a 12:12-h light/dark cycle and kept for 7 days to acclimatize before the start of the behavioural tests. Animals were fed once per 3 days with small pieces of fresh mussel meat. In behavioural tests, the startle response was induced by rapid ejection of a 10-ml syringe filled with aquarium water into actinia’s mouth opening from 1–3-cm distance. The length of a startle response was registered as a time interval between closure of actinia’s mouth with simultaneous tentacle retraction and subsequent mouth opening with tentacle straightening. In the experiments involving DZP, 100 µM solution of DZP in sea water was used. pH in both in vivo and in vitro experiments was adjusted with HCl and NaOH. Behavioural tests commenced in 1.5–2 h after pH adjustment since after a change of external pH, intracellular pH in actinia cells comes to the normal values in 35–40 min [23].
Electrophysiology
Electrophysiological recordings were performed on dissociated myoepithelial cells obtained with a protocol proposed by Holman and Anderson [15]. Briefly, after being relaxed and anesthetized in 350 mM MgCl2, the animals were dissected. The upper quarter of the actinia stem which neighbours the oral disc was cut into small pieces, then loosened with papain (3.5 mg per ml of sea water) and triturated within a syringe with an 18-gauge needle. Whole-cell patch-clamp recordings were performed in a seawater-perfused chamber at room temperature (~ 22 °C). The intracellular solution for current-clamp recordings contained (in mM) 126 K-gluconate, 8 NaCl, 5 HEPES, 15 glucose, 1 MgSO4·7H2O, 2 BAPTA, and 3 Mg-ATP, while for voltage-clamp recordings 126 CsCl, 10 KOH-HEPES, 10 BAPTA, 8 NaCl, 5 QX-314, 2 Mg-ATP, and 0.3 GTP (pH 7.2, 295 mOsm). To isolate GABAAR response in outside-out patches, we added to perfusion solution 20 µM APV (to block NMDA receptors), 10 µM NBQX (to block AMPA receptors), 50 nM CGP-55845 (to block GABAB receptors), and 1 µM strychnine (to block glycine receptors). Recordings were performed with a Multiclamp-700B amplifier and a Digidata 1550 digitizer, and the recorded traces were digitized at a 10 kHz rate and digitally filtered offline. To apply different perfusion solutions on the same membrane patch in experiments on outside-out patches, the rapid solution exchange system was used [38]. To avoid confusion due to (possible) response modification generated by uncontrolled factors, in different experiments, we used a different order of pH modifications: from lower to higher or from higher to lower values. The single receptor open-time fraction was calculated as to/tf, where to is a time in an open state and tf is a full time of recording. Automated detection of the single channel openings was performed with a threshold detection algorithm of Clampfit 11 software package, with a detection threshold set 2.5 pA more negative than a baseline conductance and a minimum event length of 2 ms.
Nonlinear fitting of concentration–response curves was performed with a Hill equation
via a Newton–Raphson iteration method, where E is an effect of GABA, C is concentration of GABA, Kd is an apparent dissociation constant, and nH is a Hill coefficient (positive for an increasing function and negative for a decreasing function).
Repeated-measures two-way analysis of variance with Geisser-Greenhouse correction for sphericity and Tukey post-hoc test (RM-ANOVA, performed with GraphPad Prism 8.0 software), nonlinear curve fitting (performed with Wolfram Mathematica 11 package) and Student’s paired t-test (performed with MS Excel) were used as indicated. GABA, QX-314, NBQX, CGP-55845, and diazepam were purchased from Tocris Bioscience, while all other chemicals were purchased from Sigma-Aldrich.
Results
At the initial stage of this project, we tested whether GABAAR functional profile in A. equina is pH-dependent and if DZP can modify GABAAR activity. To do this, we recorded in voltage-clamp mode GABAAR single-channel openings in outside-out membrane patches excised from myoepithelial cells (Fig. 1). Here and in further experiments, we did not observe a significant difference between North Sea and Black Sea actinia for any tested parameter. We thus provide combined data received from animals of both populations.
GABA and DZP activate GABAARs in A. equina cell membrane patches. A–C Example traces recorded consequently from the same membrane patch. A Test on GABAAR pharmacological specificity. From top to bottom: control (no GABAAR ligands), GABA 0.1 µM, MSC 1 µM, MSC 1 µM + PTX 20 µM. “C” and “O” indicate closed and open state of the receptor, respectively. Scale bars apply for A–C. B Ascending concentrations of GABA increase GABAAR open-time fraction. From top to bottom: GABA 0.1 µM, 1 µM, 10 µM, 100 µM. C DZP impacts GABAAR opening frequency only in presence of GABA. From top to bottom: control (no GABAAR ligands), GABA 0.1 µM, DZP 100 µM, GABA + DZP. D Concentration–response curve generated in B. Open-time fraction normalized to that obtained for 1 mM GABA. E Statistical summary for C at different pH values: pH increase upregulates GABAAR activity. Asterisks denote significance of difference from the “GABA only” effect at corresponding pH; * P < 0.05, ** P < 0.01, Student’s paired t-test, n = 6–7 pairs
At first, we tested pharmacological specificity of actinias’ GABAARs. To do this, we applied different perfusion solutions at the same membrane patch. We found that GABA (100 nM) and a specific GABAAR agonist MSC (1 µM) evoke single-channel openings, whereas GABAAR open channel blocker PTX (20 µM) shuts GABAAR openings induced by MSC (Fig. 1A). Next, we tested the concentration–response effect of GABA on actinias’ GABAARs (Fig. 1B and D). The concentration–response dependence parameters were fitted as Kd = 4.9 µM and nH = 0.66. After that, to clarify the effect of DZP, we compared an open-time fraction of recorded trace under control conditions, with 100 nM GABA, 100 µM DZP, and GABA + DZP. Neither under control conditions nor with DZP alone did we register GABAAR openings (Fig. 1C). On the contrary, the application of GABA triggered GABAAR openings and frequency increased significantly under GABA + DZP (Fig. 1C and E). This experiment was repeated with four pH values: from 7.0 to 8.5 with a 0.5 unit step. To quantify the experimental output, here and in further experiments, we used two-way RM-ANOVA with pharmacological interventions as factor 1 and pH values as factor 2.
RM-ANOVA on the open-time fraction generated the following results. Factor 1: F(1, 22) = 65.42, P < 0.0001; factor 2: F(3, 22) = 5.81, P = 0.0044; factor 1 × factor 2: F(3, 22) = 1.27, P = 0.308. Tukey test on factor 2: P = 0.0026 for pH 7.0 vs. pH 8.5, P > 0.05 for all other comparisons.
We thus found that DZP alone has no detectable effect on GABAAR in A. equina but significantly enhances the effect of GABA, as well as that actinia’s GABAAR functioning is pH-dependent.
Next, we tested the impact of GABAAR on intercellular signalling. To do this, we performed a whole-cell current-clamp recording of action potential (AP) generation in myoepithelial cells. In this experiment, we delivered standard 500 ms depolarising current injections, monitoring the modulation of the AP generation frequency caused by GABAAR ligands (GABA, DZP, GABA + DZP) and pH (Fig. 2). We found that pharmacological interventions significantly decrease the number of APs generated upon depolarising current injection, and this effect changes quantitatively under different pH levels.
Impact of GABAARs on action potential generation. A Example traces. Application of GABAAR agonists reduces the number of APs propagated upon a standard depolarising current injection. Top row: increasing GABA concentrations, recordings from the same cell. Bottom row: application of different GABAAR agonists. Scale bars apply to all traces. B, C Statistics on A. B concentration–response dependence between applied GABA and a number of APs per standard depolarising current injection; AP numbers are normalized to the value obtained in the same cell with no GABA added. Vertical axis label applies to B and C. C The number of APs generated at different pH values; number of APs obtained under pharmacological interventions normalized to control value (when no GABAAR ligands added) obtained in the same cell. Asterisks denote significance of difference from control (unity); * P < 0.05, ** P < 0.01, *** P < 0.001, Student’s paired t-test, n = 9–10 pairs
RM-ANOVA on a number of APs. Factor 1: F(1.95, 66.28) = 63.97, P < 0.0001; factor 2: F(3, 34) = 3.1, P = 0.0395; factor 1 × factor 2: F(9, 102) = 3.11, P = 0.0024. Tukey test on factor 1: P < 0.0001 for all comparisons; on factor 2: P > 0.05 for all comparisons. On top of that, to obtain more detailed characteristic of the GABA role in intercellular signalling, we next generated a concentration–response dependence for GABA’s modulatory impact on AP generation (Fig. 2). Here nonlinear fitting yielded Kd = 7.2 µM and nH = − 0.78.
Therefore, we have shown that GABAARs in A. equina modulate cell excitability in a pH-dependent manner. Equipped with this knowledge, we began to research the impact of GABAARs on A. equina behaviour.
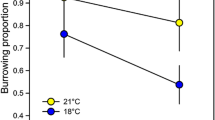
To do this, we monitored the time length of the startle response (strangulated mouth opening with retracted tentacles, see Fig. 3A) to water ejection into actinia’s mouth. Since DZP added to ejected water alone exerted a significant impact on the response length, we concluded that living animals maintain in tissues a sufficient concentration of GABA to manifest a GABAAR-related DZP effect. Here we found that DZP significantly reduces the response length under all pH levels. However, the change of pH itself did not exert a significant effect (Fig. 3B).
DZP impact on startle response. A A. equina before (left, open) and after (right, closed) water ejection into mouth opening. B Experimental statistics: startle response at different pH. Asterisks denote significance of difference from control at corresponding pH; * P < 0.05, Student’s paired t-test, n = 16 pairs
RM-ANOVA on the startle response length. Factor 1: F(1, 15) = 15.94, P = 0.0012; factor 2: F(2.8, 41.95) = 1.89, P = 0.15; factor 1 × factor 2: F(2.42, 36.48) = 0.045, P = 0.97. Tukey test on factor 2: P > 0.05 for all comparisons.
Discussion
In this research, we demonstrated for the first time the effect of a specific agonist of a GABAAR in Anthozoa at a single-receptor level and then studied the projection of such an effect to modulation of the whole cell excitatory signalling and further to the level of behaviour control. Our data confirm GABAAR to be an important factor shaping A. equina behaviour via control over AP generation machinery. The absence of DZP effect when the compound is added without GABA in vitro (Figs. 1 and 2) and presence of its effect in vivo (Fig. 3) suggest the continuous secretion of GABA by actinia’s living tissue in a concentration sufficient for activation of GABAARs.
Despite the principal role of GABAARs in the delivery of inhibitory signalling being well-established in vertebrates and in a number of invertebrate species, their functional profile in Cnidaria remains unclear. Earlier studies have reported the presence of GABA in different cnidarians [10, 27, 28] and its role as a signalling molecule in feeding behaviour, orientation, tentacle movement, and so on [17, 18, 34]. Pharmacological data suggest the presence of GABAARs in Cnidaria [33]: an observation that was later supported by genetic evidence [1]. However, the structure of the GABAAR derived from the genome of the sea anemone Nematostella vectensis [1] differs substantially from that suggested by the pharmacological profile of Hydra vulgaris receptors [9, 33], with the latter being similar to vertebrate orthologues. Our data demonstrate the inhibitory effect of DZP, given that it is a specific allosteric agonist of vertebrate GABAARs. However, despite DZP being shown to upregulate the GABA-independent activation of vertebrate GABAARs previously [3], in our work, we did not observe any significant effect of DZP in the absence of GABA (Fig. 1C). Apart from that and on the contrary to common observations in vertebrates [3], in our recordings from outside-out patches, we failed to register spontaneous GABAAR openings (Fig. 1A and C, top traces). Our observations thus give indirect support to the hypothesis of significant structural difference between GABAARs in vertebrates and in Anthozoa [1].
An important issue arising from the data collected in our work is indeed why fluctuations of pH exert a significant effect on the functional profile of a single GABAAR (Fig. 1) and on GABAAR-mediated control over AP-generating mechanisms in a single cell (Fig. 2) but not on GABAAR-mediated behavioural reactions of living animals (Fig. 3). The low sensitivity of the startle response to GABAAR effects is an unlikely reason for this since the startle response experienced a highly significant impact from GABAAR-mediated effect of DZP. A plausible explanation is a loss of the GABAAR response sensitivity to pH at saturative concentrations of GABA [8, 36] since the protonation of GABAAR extracellular domain modulates GABA binding kinetics [30]. To the best of our knowledge, the steady-state concentration of GABA in A. equina tissue, as well as the binding constant(s) of GABAARs subspecies expressed in actinia, has not yet been determined. Therefore, at present we have insufficient data to make a meaningful conclusion as to how close to a saturative level the native GABA concentration maintained in actinia tissues is. However, in vertebrates’ neural tissue, the native concentration of GABA was reported to be of micromolar range [22, 25, 31], which is more than an order of magnitude higher than 100-nM concentration used in our in vitro experiments, where the effect of pH was statistically significant. Additionally, to the best of our knowledge, there are no data on the share of actinia’s GABAARs localized in synapses vs. at extrasynaptic cell membranes. Taking into account that the concentration of GABA released into synaptic cleft is commonly 3–5 orders of magnitude higher than in extracellular space and far exceeds the level of saturation of GABAARs [13], a high “synaptic/extrasynaptic” ratio of GABAARs’ distribution in actinia’s excitable cells may support resistance to pH fluctuations.
By exploring the pH interval from 7.0 to 8.5 in our experiments, we have covered the full range of inter-annual pH fluctuations observed in the North Sea and Black Sea at present (which is 7.5–8.5) [4, 35] plus maximum predicted acidification (pH value decrease by 0.45 unit) [7, 39]). Hence, our data from behavioural tests (Fig. 3) suggest A. equina to be well-prepared for any OA scenario: actinia’s nervous system stability and behavioural patterns should not be affected by the predicted pH shift.
Abbreviations
- AP:
-
Action potential
- APV:
-
D-2-amino-5-phosphonovalerate
- ASAB:
-
Association for study of animal behaviour
- CGP-55845:
-
(2S)-3-[[(1S)-1-(3,4-Dichlorophenyl)ethyl]-amino-2-hydroxypropyl](phenylmethyl)phosphinic acid
- DZP:
-
Diazepam
- GABA:
-
γ-Aminobutyric acid
- GABAAR:
-
GABA receptor of type A
- GABABR:
-
GABA receptor of type B
- MSC:
-
Muscimol
- NBQX:
-
2,3-Dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide
- OA:
-
Ocean acidification
- PTX:
-
Picrotoxin
- RM-ANOVA:
-
Repeated-measures analysis of variance
References
Anctil M (2009) Chemical transmission in the sea anemone Nematostella vectensis: a genomic perspective. Comp Biochem Physiol Part D Genomics Proteomics 4:268–289. https://doi.org/10.1016/j.cbd.2009.07.001
Avsar NB, Jin S, Kutoglu H, Gurbuz G (2016) Sea level change along the Black Sea coast from satellite altimetry, tide gauge and GPS observations. Geodesy and Geodynamics 7:50–55. https://doi.org/10.1016/j.geog.2016.03.005
Birnir B, Eghbali M, Everitt AB, Gage PW (2000) Bicuculline, pentobarbital and diazepam modulate spontaneous GABAA channels in rat hippocampal neurons. Br J Pharmacol 131:695–704. https://doi.org/10.1038/sj.bjp.0703621
Blackford JC, Gilbert FJ (2007) pH variability and CO2 induced acidification in the North Sea. J Mar Syst 64:229–241. https://doi.org/10.1016/j.jmarsys.2006.03.016
Briffa M, Greenaway J (2011) High in situ repeatability of behaviour indicates animal personality in the beadlet anemone Actinia equina (Cnidaria). PLoS ONE 6:e21963. https://doi.org/10.1371/journal.pone.0021963
Buchanan K, Burt de Perera T, Carere C, Carter T, Hailey A, Hubrecht R, Jennings D, Metcalfe N, Pitcher T, Peron F (2012) Guidelines for the treatment of animals in behavioural research and teaching. Anim Behav 83:301–309
Caldeira K, Wickett ME (2005) Ocean model predictions of chemistry changes from carbon dioxide emissions to the atmosphere and ocean. J Geophys Res Oceans 110. https://doi.org/10.1029/2004JC002671
Chen ZL, Huang RQ (2014) Extracellular pH modulates GABAergic neurotransmission in rat hypothalamus. Neuroscience 271:64–76. https://doi.org/10.1016/j.neuroscience.2014.04.028
Concas A, Pierobon P, Mostallino MC, Marino G, Minei R, Biggio G (1998) Modulation of γ-aminobutyric acid (GABA) receptors and the feeding response by neurosteroids in Hydra vulgaris. Neuroscience 85:979–988. https://doi.org/10.1016/S0306-4522(97)00515-0
Delgado LM, Couve E, Schmachtenberg O (2010) GABA and glutamate immunoreactivity in tentacles of the sea anemone Phymactis papillosa (LESSON 1830). J Morphol 271:845–852. https://doi.org/10.1002/jmor.10838
Foster NL, Briffa M (2014) Familial strife on the seashore: aggression increases with relatedness in the sea anemone Actinia equina. Behav Proc 103:243–245. https://doi.org/10.1016/j.beproc.2014.01.009
Gojobori T, Ikeo K, Hwang JS (2009) The evolutionary origin and process of the central nervous system: comparative genomics approach. Scientific Insights into the Evolution of the Universe and of Life. Pontifical Academy of Sciences
Grabauskas G (2004) Time course of GABA in the synaptic clefts of inhibitory synapses in the rostral nucleus of the solitary tract. Neurosci Lett 373:10–15. https://doi.org/10.1016/j.neulet.2004.09.051
Hoegh-Guldberg O, Bruno JF (2010) The impact of climate change on the world’s marine ecosystems. Science 328:1523–1528. https://doi.org/10.1126/science.1189930
Holman MA, Anderson PAV (1991) Voltage-activated ionic currents in myoepithelial cells isolated from the sea anemone Calliactis tricolor. J Exp Biol 161:333–346
Jarrold MD, Calosi P, Verberk WCEP, Rastrick SPS, Atfield A, Spicer JI (2013) Physiological plasticity preserves the metabolic relationship of the intertidal non-calcifying anthozoan-Symbiodinium symbiosis under ocean acidification. J Exp Mar Biol Ecol 449:200–206. https://doi.org/10.1016/j.jembe.2013.09.013
Kass-Simon G, Pannaccione A, Pierobon P (2003) GABA and glutamate receptors are involved in modulating pacemaker activity in hydra. Comp Biochem Physiol A Mol Integr Physiol 136:329–342. https://doi.org/10.1016/s1095-6433(03)00168-5
Kass-Simon G, Scappaticci AA (2004) Glutamatergic and GABAnergic control in the tentacle effector systems of Hydra vulgaris. Hydrobiologia 530:67–71. https://doi.org/10.1007/s10750-004-2647-7
Kass-Simon G, ScappaticciAA J (2002) The behavioral and developmental physiology of nematocysts. Can J Zool 80:1772–1794
Klein SG, Pitt KA, Nitschke MR, Goyen S, Welsh DT, Suggett DJ, Carroll AR (2017) Symbiodinium mitigate the combined effects of hypoxia and acidification on a noncalcifying cnidarian. Glob Change Biol 23:3690–3703. https://doi.org/10.1111/gcb.13718
Krishek BJ, Amato A, Connolly CN, Moss SJ, Smart TG (1996) Proton sensitivity of the GABA(A) receptor is associated with the receptor subunit composition. J Physiol 492(Pt 2):431–443. https://doi.org/10.1113/jphysiol.1996.sp021319
Kuntz A, Clement HW, Lehnert W, van Calker D, Hennighausen K, Gerlach M, Schulz E (2004) Effects of secretin on extracellular amino acid concentrations in rat hippocampus. J Neural Transm (Vienna) 111:931–939. https://doi.org/10.1007/s00702-003-0082-y
Laurent J, Venn A, Tambutté É, Ganot P, Allemand D, Tambutté S (2014) Regulation of intracellular pH in cnidarians: response to acidosis in Anemonia viridis. Febs j 281:683–695. https://doi.org/10.1111/febs.12614
Le Bon O, Basiaux P, Streel E, Tecco J, Hanak C, Hansenne M, Ansseau M, Pelc I, Verbanck P, Dupont S (2004) Personality profile and drug of choice; a multivariate analysis using Cloninger’s TCI on heroin addicts, alcoholics, and a random population group. Drug Alcohol Depend 73:175–182. https://doi.org/10.1016/j.drugalcdep.2003.10.006
Lerma J, Herranz AS, Herreras O, Abraira V, Martín del Río R (1986) In vivo determination of extracellular concentration of amino acids in the rat hippocampus. A method based on brain dialysis and computerized analysis. Brain Res 384:145–155. https://doi.org/10.1016/0006-8993(86)91230-8
Maltby L (1999) Studying stress: the importance of organism-level responses. Ecol Appl 9:431–440. https://doi.org/10.1890/1051-0761(1999)009[0431:SSTIOO]2.0.CO;2
Marlow HQ, Srivastava M, Matus DQ, Rokhsar D, Martindale MQ (2009) Anatomy and development of the nervous system of Nematostella vectensis, an anthozoan cnidarian. Dev Neurobiol 69:235–254. https://doi.org/10.1002/dneu.20698
Martin VJ (2004) Photoreceptors of cubozoan jellyfish. Hydrobiologia 530:135–144. https://doi.org/10.1007/s10750-004-2674-4
Maskrey DK, Sneddon LU, Arnold KE, Wolfenden DCC, Thomson JS (2020) The impact of personality, morphotype and shore height on temperature-mediated behavioural responses in the beadlet anemone Actinia equina. J Anim Ecol. https://doi.org/10.1111/1365-2656.13301
Mozrzymas JW, Żarmowska ED, Pytel M, Mercik K (2003) Modulation of GABAA receptors by hydrogen ions reveals synaptic GABA transient and a crucial role of the desensitization process. J Neurosci 23:7981–7992. https://doi.org/10.1523/jneurosci.23-22-07981.2003
Nyitrai G, Kékesi KA, Juhász G (2006) Extracellular level of GABA and Glu: in vivo microdialysis-HPLC measurements. Curr Top Med Chem 6:935–940. https://doi.org/10.2174/156802606777323674
Pierobon P (2012) Coordinated modulation of cellular signaling through ligand-gated ion channels in Hydra vulgaris (Cnidaria, Hydrozoa). Int J Dev Biol 56:551–565. https://doi.org/10.1387/ijdb.113464pp
Pierobon P, Concas A, Santoro G, Marino G, Minei R, Pannaccione A, Mostallino MC, Biggio G (1995) Biochemical and functional identification of GABA receptors in Hydra vulgaris. Life Sci 56:1485–1497. https://doi.org/10.1016/0024-3205(95)00111-I
Pierobon P, Tino A, Minei R, Marino G (2004) Different roles of GABA and glycine in the modulation of chemosensory responses in Hydra vulgaris (Cnidaria, Hydrozoa). Hydrobiologia 530:59–66. https://doi.org/10.1007/s10750-004-2690-4
Polonsky A (2012) Had been observing the acidification of the Black Sea upper layer in XX century? Turk J Fish Aquat Sci 12:391–396
Robello M, Baldelli P, Cupello A (1994) Modulation by extracellular pH of the activity of GABAA receptors on rat cerebellum granule cells. Neuroscience 61:833–837. https://doi.org/10.1016/0306-4522(94)90406-5
Snigirov S, Chernyavskiy A, Naum E, Galkina A, Medinets V, Gazyetov YI, Konareva O, Snigirov P (2019) Zmiinyi Island Coastal Zone Macrozoobenthos State in 2016–2017 (in Russian). Visnyk of V N Karazin Kharkiv National University Series "Ecology" 81–98. https://doi.org/10.26565/1992-4259-2019-21-07
Sylantyev S, Rusakov DA (2013) Sub-millisecond ligand probing of cell receptors with multiple solution exchange. Nat Protoc 8:1299–1306. https://doi.org/10.1038/nprot.2013.075
Turley C, Eby M, Ridgwell AJ, Schmidt DN, Findlay HS, Brownlee C, Riebesell U, Fabry VJ, Feely RA, Gattuso JP (2010) The societal challenge of ocean acidification. Mar Pollut Bull 60:787–792. https://doi.org/10.1016/j.marpolbul.2010.05.006
Urbarova I, Forêt S, Dahl M, Emblem Å, Milazzo M, Hall-Spencer JM, Johansen SD (2019) Ocean acidification at a coastal CO2 vent induces expression of stress-related transcripts and transposable elements in the sea anemone Anemonia viridis. PloS one 14:e0210358
Vine R (1994) Benzodiazepine use by women prisoners: association with personality disorder and behavioural dyscontrol. Psychiatry Psychol Law 1:53–58. https://doi.org/10.1080/13218719409524828
Voshaar RCO, Gorgels WJMJ, Mol AJJ, Van Balkom AJLM, Van De Lisdonk EH, Breteler MHM, Van Den Hoogen JM, Zitman FG (2003) Tapering off long-term benzodiazepine use with or without group cognitive-behavioural therapy: three-condition, randomised controlled trial. Br J Psychiatry 182:498–504. https://doi.org/10.1192/bjp.182.6.498
Zhou C, Xiao C, Deng C, Hong Ye J (2007) Extracellular proton modulates GABAergic synaptic transmission in rat hippocampal CA3 neurons. Brain Res 1145:213–220. https://doi.org/10.1016/j.brainres.2007.01.121
Acknowledgements
This study was supported with Wellcome-ISSF award via the University of Aberdeen and the University of Aberdeen Pump-Priming award SF10237-52 for S. Sylantyev and with Improving Environmental Monitoring in the Black Sea – Phase II (EMBLAS-II) ENPI/2013/313-169 grant for S. Snigirov.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Snigirov, S., Sylantyev, S. The regulatory role of GABAA receptor in Actinia equina nervous system and the possible effect of global ocean acidification. Pflugers Arch - Eur J Physiol 473, 1851–1858 (2021). https://doi.org/10.1007/s00424-021-02628-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-021-02628-w