Abstract
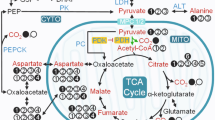
The role of pyruvate dehydrogenase in mediating lipid-induced insulin resistance stands as a central question in the pathogenesis of type 2 diabetes mellitus. Many researchers have invoked the Randle hypothesis to explain the reduced glucose disposal in skeletal muscle by envisioning an elevated acetyl CoA pool arising from increased oxidation of fatty acids. Over the years, in vivo NMR studies have challenged that monolithic view. The advent of the dissolution dynamic nuclear polarization NMR technique and a unique type 2 diabetic rat model provides an opportunity to clarify. Dynamic nuclear polarization enhances dramatically the NMR signal sensitivity and allows the measurement of metabolic kinetics in vivo. Diabetic muscle has much lower pyruvate dehydrogenase activity than control muscle, as evidenced in the conversion of [1-13C]lactate and [2-13C]pyruvate to HCO3− and acetyl carnitine. The pyruvate dehydrogenase kinase inhibitor, dichloroacetate, restores rapidly the diabetic pyruvate dehydrogenase activity to control level. However, diabetic muscle has a much larger dynamic change in pyruvate dehydrogenase flux than control. The dichloroacetate-induced surge in pyruvate dehydrogenase activity produces a differential amount of acetyl carnitine but does not affect the tricarboxylic acid flux. Further studies can now proceed with the dynamic nuclear polarization approach and a unique rat model to interrogate closely the biochemical mechanism interfacing oxidative metabolism with insulin resistance and metabolic inflexibility.






Similar content being viewed by others
Availability of data and material
All data generated or analyzed during this study are included in this published article.
Abbreviations
- IRS:
-
Insulin receptor substrate
- IMCL:
-
Intramuscular lipid
- T2DM:
-
Type 2 diabetes mellitus
- DNP:
-
Dynamic nuclear polarization
- PDH:
-
Pyruvate dehydrogenase
- DCA:
-
Dichloroacetate
- PFK:
-
Phosphofructokinase
- G6P:
-
Glucose 6 phosphate
- PGC-1α:
-
Peroxisome proliferator-activated receptor-γ coactivator 1α
- PDK:
-
Pyruvate dehydrogenase kinase
- DKO:
-
Double knock-out
- ETC:
-
Electron transport chain
- tC:
-
Total 13C signal
- ALCAR:
-
Acetyl carnitine
- AcAc:
-
Acetoacetate
- LDH:
-
Lactate dehydrogenase
- ALT:
-
Alanine aminotransferase
- TCA:
-
Tricarboxylic acid
- CrAT:
-
Carnitine acetyl transferase
References
Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K (2003) Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc Natl Acad Sci U S A 100:10158–10163. https://doi.org/10.1073/pnas.1733835100
Atherton HJ, Schroeder MA, Dodd MS, Heather LC, Carter EE, Cochlin LE, Nagel S, Sibson NR, Radda GK, Clarke K, Tyler DJ (2011) Validation of the in vivo assessment of pyruvate dehydrogenase activity using hyperpolarised 13C MRS. NMR Biomed 24:201–208. https://doi.org/10.1002/nbm.1573
Backshear PJ, Holloway PA, Alberti KG (1975) Metabolic interactions of dichloroacetate and insulin in experimental diabetic ketoacidosis. Biochem J 146:447–456. https://doi.org/10.1042/bj1460447
Bastiaansen JAM, Yoshihara HAL, Takado Y, Gruetter R, Comment A (2014) Hyperpolarized 13C lactate as a substrate for in vivo metabolic studies in skeletal muscle. Metabolomics 10:986–994. https://doi.org/10.1007/s11306-014-0630-5
Befroy DE, Petersen KF, Dufour S, Mason GF, de Graaf RA, Rothman DL, Shulman GI (2007) Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes 56:1376–1381. https://doi.org/10.2337/db06-0783
Boushel R, Gnaiger E, Schjerling P, Skovbro M, Kraunsoe R, Dela F (2007) Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia 50:790–796. https://doi.org/10.1007/s00125-007-0594-3
Bowker-Kinley MM, Davis WI, Wu P, Harris RA, Popov KM (1998) Evidence for existence of tissue-specific regulation of the mammalian pyruvate dehydrogenase complex. Biochem J 329(Pt 1):191–196. https://doi.org/10.1042/bj3290191
Brooks GA (2009) Cell-cell and intracellular lactate shuttles. J Physiol 587:5591–5600. https://doi.org/10.1113/jphysiol.2009.178350
Cline GW, Lepine RL, Papas KK, Kibbey RG, Shulman GI (2004) 13C NMR isotopomer analysis of anaplerotic pathways in INS-1 cells. J Biol Chem 279:44370–44375. https://doi.org/10.1074/jbc.M311842200
Cummings BP, Bettaieb A, Graham JL, Stanhope KL, Dill R, Morton GJ, Haj FG, Havel PJ (2011) Subcutaneous administration of leptin normalizes fasting plasma glucose in obese type 2 diabetic UCD-T2DM rats. Proc Natl Acad Sci U S A 108:14670–14675. https://doi.org/10.1073/pnas.1107163108
Cummings BP, Digitale EK, Stanhope KL, Graham JL, Baskin DG, Reed BJ, Sweet IR, Griffen SC, Havel PJ (2008) Development and characterization of a novel rat model of type 2 diabetes mellitus: the UC Davis type 2 diabetes mellitus UCD-T2DM rat. Am J Physiol Regul Integr Comp Physiol 295:R1782–R1793. https://doi.org/10.1152/ajpregu.90635.2008
Day SE, Kettunen MI, Gallagher FA, Hu DE, Lerche M, Wolber J, Golman K, Ardenkjaer-Larsen JH, Brindle KM (2007) Detecting tumor response to treatment using hyperpolarized 13C magnetic resonance imaging and spectroscopy. Nat Med 13:1382–1387. https://doi.org/10.1038/nm1650
Grassi B, Gladden LB, Samaja M, Stary CM, Hogan MC (1998) Faster adjustment of O2 delivery does not affect V(O2) on-kinetics in isolated in situ canine muscle. J Appl Physiol 85:1394–1403. https://doi.org/10.1152/jappl.1998.85.4.1394
He J, Watkins S, Kelley DE (2001) Skeletal muscle lipid content and oxidative enzyme activity in relation to muscle fiber type in type 2 diabetes and obesity. Diabetes 50:817–823
Hesselink MK, Schrauwen-Hinderling V, Schrauwen P (2016) Skeletal muscle mitochondria as a target to prevent or treat type 2 diabetes mellitus. Nat Rev Endocrinol 12:633–645. https://doi.org/10.1038/nrendo.2016.104
Holness MJ, Kraus A, Harris RA, Sugden MC (2000) Targeted upregulation of pyruvate dehydrogenase kinase (PDK)-4 in slow-twitch skeletal muscle underlies the stable modification of the regulatory characteristics of PDK induced by high-fat feeding. Diabetes 49:775–781. https://doi.org/10.2337/diabetes.49.5.775
Howlett RA, Heigenhauser GJ, Hultman E, Hollidge-Horvat MG, Spriet LL (1999) Effects of dichloroacetate infusion on human skeletal muscle metabolism at the onset of exercise. Am J Physiol 277:E18–E25
Hue L, Taegtmeyer H (2009) The Randle cycle revisited: a new head for an old hat. Am J Physiol Endocrinol Metab 297:E578-591. https://doi.org/10.1152/ajpendo.00093.2009
Jeoung NH, Harris RA (2008) Pyruvate dehydrogenase kinase-4 deficiency lowers blood glucose and improves glucose tolerance in diet-induced obese mice. Am J Physiol Endocrinol Metab 295:E46-54. https://doi.org/10.1152/ajpendo.00536.2007
Kautzky-Willer A, Krssak M, Winzer C, Pacini G, Tura A, Farhan S, Wagner O, Brabant G, Horn R, Stingl H, Schneider B, Waldhausl W, Roden M (2003) Increased intramyocellular lipid concentration identifies impaired glucose metabolism in women with previous gestational diabetes. Diabetes 52:244–251
Kelley DE, Goodpaster BH, Storlien L (2002) Muscle triglyceride and insulin resistance. Annu Rev Nutr 22:325–346. https://doi.org/10.1146/annurev.nutr.22.010402.102912
Kelley DE, He J, Menshikova EV, Ritov VB (2002) Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51:2944–2950. https://doi.org/10.2337/diabetes.51.10.2944
Kelley DE, Mandarino LJ (2000) Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes 49:677–683. https://doi.org/10.2337/diabetes.49.5.677
Kemp GJ, Brindle KM (2012) What do magnetic resonance-based measurements of Pi–>ATP flux tell us about skeletal muscle metabolism? Diabetes 61:1927–1934. https://doi.org/10.2337/db11-1725
Kettunen MI, Hu DE, Witney TH, McLaughlin R, Gallagher FA, Bohndiek SE, Day SE, Brindle KM (2010) Magnetization transfer measurements of exchange between hyperpolarized [1-13C]pyruvate and [1-13C]lactate in a murine lymphoma. Magn Reson Med 63:872–880. https://doi.org/10.1002/mrm.22276
Kleinert M, Clemmensen C, Hofmann SM, Moore MC, Renner S, Woods SC, Huypens P, Beckers J, de Angelis MH, Schurmann A, Bakhti M, Klingenspor M, Heiman M, Cherrington AD, Ristow M, Lickert H, Wolf E, Havel PJ, Muller TD, Tschop MH (2018) Animal models of obesity and diabetes mellitus. Nat Rev Endocrinol 14:140–162. https://doi.org/10.1038/nrendo.2017.161
Koliaki C, Roden M (2016) Alterations of mitochondrial function and insulin sensitivity in human obesity and diabetes mellitus. Annu Rev Nutr 36:337–367. https://doi.org/10.1146/annurev-nutr-071715-050656
Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM (2008) Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7:45–56. https://doi.org/10.1016/j.cmet.2007.10.013
Le Page LM, Rider OJ, Lewis AJ, Ball V, Clarke K, Johansson E, Carr CA, Heather LC, Tyler DJ (2015) Increasing pyruvate dehydrogenase flux as a treatment for diabetic cardiomyopathy: a combined 13C hyperpolarized magnetic resonance and echocardiography study. Diabetes 64:2735–2743. https://doi.org/10.2337/db14-1560
Lindeboom L, Nabuurs CI, Hoeks J, Brouwers B, Phielix E, Kooi ME, Hesselink MK, Wildberger JE, Stevens RD, Koves T, Muoio DM, Schrauwen P, Schrauwen-Hinderling VB (2014) Long-echo time MR spectroscopy for skeletal muscle acetylcarnitine detection. J Clin Invest 124:4915–4925. https://doi.org/10.1172/JCI74830
Madiraju AK, Erion DM, Rahimi Y, Zhang XM, Braddock DT, Albright RA, Prigaro BJ, Wood JL, Bhanot S, MacDonald MJ, Jurczak MJ, Camporez JP, Lee HY, Cline GW, Samuel VT, Kibbey RG, Shulman GI (2014) Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 510:542–546. https://doi.org/10.1038/nature13270
Madiraju AK, Qiu Y, Perry RJ, Rahimi Y, Zhang XM, Zhang D, Camporez JG, Cline GW, Butrico GM, Kemp BE, Casals G, Steinberg GR, Vatner DF, Petersen KF, Shulman GI (2018) Metformin inhibits gluconeogenesis via a redox-dependent mechanism in vivo. Nat Med 24:1384–1394. https://doi.org/10.1038/s41591-018-0125-4
Mayers RM, Leighton B, Kilgour E (2005) PDH kinase inhibitors: a novel therapy for Type II diabetes? Biochem Soc Trans 33:367–370. https://doi.org/10.1042/BST0330367
Mogensen M, Sahlin K, Fernstrom M, Glintborg D, Vind BF, Beck-Nielsen H, Hojlund K (2007) Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes 56:1592–1599. https://doi.org/10.2337/db06-0981
Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC (2003) PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34:267–273. https://doi.org/10.1038/ng1180
Noland RC, Koves TR, Seiler SE, Lum H, Lust RM, Ilkayeva O, Stevens RD, Hegardt FG, Muoio DM (2009) Carnitine insufficiency caused by aging and overnutrition compromises mitochondrial performance and metabolic control. J Biol Chem 284:22840–22852. https://doi.org/10.1074/jbc.M109.032888
Ortenblad N, Mogensen M, Petersen I, Hojlund K, Levin K, Sahlin K, Beck-Nielsen H, Gaster M (2005) Reduced insulin-mediated citrate synthase activity in cultured skeletal muscle cells from patients with type 2 diabetes: evidence for an intrinsic oxidative enzyme defect. Biochim Biophys Acta 1741:206–214. https://doi.org/10.1016/j.bbadis.2005.04.001
Park JM, Harrison CE, Ma J, Chen J, Ratnakar J, Zun Z, Liticker J, Reed GD, Chhabra A, Haller RG, Jue T, Malloy CR (2021) Hyperpolarized (13)C MR spectroscopy depicts in vivo effect of exercise on pyruvate metabolism in human skeletal muscle. Radiology:204500. https://doi.org/10.1148/radiol.2021204500
Park JM, Josan S, Mayer D, Hurd R, Spielman D, Bendahan D, Jue T (2013) Direct observation of lactate metabolsim in skeletal muscle with hyperpolarized 13C NMR. World Molecular Imaging Congress:LBA 9
Park JM, Josan S, Mayer D, Hurd RE, Chung Y, Bendahan D, Spielman DM, Jue T (2015) Hyperpolarized 13C NMR observation of lactate kinetics in skeletal muscle. J Exp Biol 218:3308–3318. https://doi.org/10.1242/jeb.123141
Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ (2003) Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A 100:8466–8471. https://doi.org/10.1073/pnas.1032913100
Patti ME, Corvera S (2010) The role of mitochondria in the pathogenesis of type 2 diabetes. Endocr Rev 31:364–395. https://doi.org/10.1210/er.2009-0027
Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI (2003) Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300:1140–1142. https://doi.org/10.1126/science.1082889
Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI (2004) Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 350:664–671. https://doi.org/10.1056/NEJMoa031314
Petersen KF, Dufour S, Shulman GI (2005) Decreased insulin-stimulated ATP synthesis and phosphate transport in muscle of insulin-resistant offspring of type 2 diabetic parents. PLoS Med 2:e233. https://doi.org/10.1371/journal.pmed.0020233
Petersen KF, Morino K, Alves TC, Kibbey RG, Dufour S, Sono S, Yoo PS, Cline GW, Shulman GI (2015) Effect of aging on muscle mitochondrial substrate utilization in humans. Proc Natl Acad Sci U S A 112:11330–11334. https://doi.org/10.1073/pnas.1514844112
Petersen MC, Shulman GI (2018) Mechanisms of insulin action and insulin resistance. Physiol Rev 98:2133–2223. https://doi.org/10.1152/physrev.00063.2017
Pieklik JR, Guynn RW (1975) Equilibrium constants of the reactions of choline acetyltransferase, carnitine acetyltransferase, and acetylcholinesterase under physiological conditions. J Biol Chem 250:4445–4450
Power RA, Hulver MW, Zhang JY, Dubois J, Marchand RM, Ilkayeva O, Muoio DM, Mynatt RL (2007) Carnitine revisited: potential use as adjunctive treatment in diabetes. Diabetologia 50:824–832. https://doi.org/10.1007/s00125-007-0605-4
Rahimi Y, Camporez JP, Petersen MC, Pesta D, Perry RJ, Jurczak MJ, Cline GW, Shulman GI (2014) Genetic activation of pyruvate dehydrogenase alters oxidative substrate selection to induce skeletal muscle insulin resistance. Proc Natl Acad Sci U S A 111:16508–16513. https://doi.org/10.1073/pnas.1419104111
Randle PJ, Garland PB, Hales CN, Newsholme EA (1963) The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1:785–789. https://doi.org/10.1016/s0140-6736(63)91500-9
Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, Shulman GI (1996) Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest 97:2859–2865. https://doi.org/10.1172/JCI118742
Rothman DL, Shulman RG, Shulman GI (1992) 31P nuclear magnetic resonance measurements of muscle glucose-6-phosphate. Evidence for reduced insulin-dependent muscle glucose transport or phosphorylation activity in non-insulin-dependent diabetes mellitus. J Clin Invest 89:1069–1075. https://doi.org/10.1172/JCI115686
Samuel VT, Petersen KF, Shulman GI (2010) Lipid-induced insulin resistance: unravelling the mechanism. Lancet 375:2267–2277. https://doi.org/10.1016/S0140-6736(10)60408-4
Samuel VT, Shulman GI (2016) The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J Clin Invest 126:12–22. https://doi.org/10.1172/JCI77812
Schroeder MA, Ali MA, Hulikova A, Supuran CT, Clarke K, Vaughan-Jones RD, Tyler DJ, Swietach P (2013) Extramitochondrial domain rich in carbonic anhydrase activity improves myocardial energetics. Proc Natl Acad Sci U S A 110:E958-967. https://doi.org/10.1073/pnas.1213471110
Schroeder MA, Cochlin LE, Heather LC, Clarke K, Radda GK, Tyler DJ (2008) In vivo assessment of pyruvate dehydrogenase flux in the heart using hyperpolarized carbon-13 magnetic resonance. Proc Natl Acad Sci U S A 105:12051–12056. https://doi.org/10.1073/pnas.0805953105
Shulman GI, Rossetti L, Rothman DL, Blair JB, Smith D (1987) Quantitative analysis of glycogen repletion by nuclear magnetic resonance spectroscopy in the conscious rat. J Clin Invest 80:387–393. https://doi.org/10.1172/JCI113084
Shulman GI, Rothman DL, Jue T, Stein P, DeFronzo RA, Shulman RG (1990) Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med 322:223–228
Simoneau JA (1985) Kelley DE (1997) Altered glycolytic and oxidative capacities of skeletal muscle contribute to insulin resistance in NIDDM. J Appl Physiol 83:166–171. https://doi.org/10.1152/jappl.1997.83.1.166
Spriet LL, Heigenhauser GJ (2002) Regulation of pyruvate dehydrogenase (PDH) activity in human skeletal muscle during exercise. Exerc Sport Sci Rev 30:91–95. https://doi.org/10.1097/00003677-200204000-00009
Stacpoole PW (1989) The pharmacology of dichloroacetate. Metabolism 38:1124–1144. https://doi.org/10.1016/0026-0495(89)90051-6
Stacpoole PW, Moore GW, Kornhauser DM (1978) Metabolic effects of dichloroacetate in patients with diabetes mellitus and hyperlipoproteinemia. 298:526–530. https://doi.org/10.1016/0026-0495(89)90051-6
Sugden MC, Holness MJ, Palmer TN (1989) Fuel selection and carbon flux during the starved-to-fed transition. Biochem J 263:313–323. https://doi.org/10.1042/bj2630313
Szendroedi J, Schmid AI, Chmelik M, Toth C, Brehm A, Krssak M, Nowotny P, Wolzt M, Waldhausl W, Roden M (2007) Muscle mitochondrial ATP synthesis and glucose transport/phosphorylation in type 2 diabetes. PLoS Med 4:e154. https://doi.org/10.1371/journal.pmed.0040154
Timmons JA, Poucher SM, Constantin-Teodosiu D, Worrall V, Macdonald IA, Greenhaff PL (1996) Increased acetyl group availability enhances contractile function of canine skeletal muscle during ischemia. J Clin Invest 97:879–883. https://doi.org/10.1172/JCI118490
Tschakovsy ME, Hughson RL (1999) Interaction of factors determining oxygen uptake at the onset of exercise. J Appl 86:1101–1113. https://doi.org/10.1152/jappl.1999.86.4.1101
Vondra K, Rath R, Bass A, Slabochova Z, Teisinger J, Vitek V (1977) Enzyme activities in quadriceps femoris muscle of obese diabetic male patients. Diabetologia 13:527–529. https://doi.org/10.1007/BF01234508
Zhang S, Hulver MW, McMillan RP, Cline MA, Gilbert ER (2014) The pivotal role of pyruvate dehydrogenase kinases in metabolic flexibility. Nutr Metab (Lond) 11:10. https://doi.org/10.1186/1743-7075-11-10
Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE (2001) Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108:1167–1174. https://doi.org/10.1172/JCI13505
Funding
The study acknowledges funding support from the National Institutes of Health (NIH) CA176836, AA005965, AA018681, S10 OD012283, P41 EB015891 (DS); the US Department of Defense PC100427 (DS); NIH EB009070, DK106395, NS096575, CA213020 (DM); NIH R01NS107409 (JMP); the Welch Foundation I-2009–20190330 (JMP); DK095960, DK087307 (PH); the France-Berkeley Fund (TJ & DB); and California Department of Public Health 18–10923 (TJ).
Author information
Authors and Affiliations
Contributions
JMP, REH, DM, DMS, and TJ contributed to the conception and design of research; JMP, SJ, REH, JG, DB, and TJ performed experiments; JMP, SJ, REH, JG, PH, DB, DM, YC, DMS, and TJ discussed, analyzed, and interpreted experiment data; JMP and TJ prepared figures, analyzed the data, wrote the drafts of the manuscript, and incorporated comments; JMP, SJ, REH, JG, PH, DB, DM, YC, DMS, and TJ reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Animal care and experimental procedures followed the guidelines of the National Institute of Health Office for Laboratory Animal Welfare and were approved by the local Institutional Animal Care and Use Committee.
Conflict of interest
The authors declare no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Park, J.M., Josan, S., Hurd, R.E. et al. Hyperpolarized NMR study of the impact of pyruvate dehydrogenase kinase inhibition on the pyruvate dehydrogenase and TCA flux in type 2 diabetic rat muscle. Pflugers Arch - Eur J Physiol 473, 1761–1773 (2021). https://doi.org/10.1007/s00424-021-02613-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-021-02613-3