Abstract
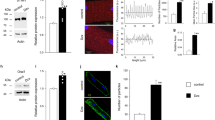
Mitochondria are important sites for the production of ATP and the generation of ROS in cells. However, whether acute hypoxia increases ROS generation in cells or affects ATP production remains unclear, and therefore, monitoring the changes in ATP and ROS in living cells in real time is important. In this study, cardiomyocytes were transfected with RoGFP for ROS detection and MitGO-Ateam2 for ATP detection, whereby ROS and ATP production in cardiomyocytes were respectively monitored in real time. Furthermore, the oxygen consumption rate (OCR) of cardiomyocytes was measured. Similar results were produced for adult and neonatal rat cardiomyocytes. Hypoxia (1% O2) reduced the basal OCR, ATP-linked OCR, and maximal OCR in cardiomyocytes compared with these OCR levels in the cardiomyocytes in the normoxic group (21% O2). However, ATP-linked OCR, normalized to maximal OCR, was increased during hypoxia, indicating that the electron leakage of complex III exacerbated the increase of ATP-linked oxygen consumption during hypoxia and vice versa. Combined with the result that cardiomyocytes expressing MitGO-Ateam2 showed a significant decrease in ATP production during hypoxia compared with that of normoxic group, acute hypoxia might depress the mitochondrial oxygen utilization efficiency of the cardiomyocytes. Moreover, cardiomyocytes expressing Cyto-RoGFP or IMS-RoGFP showed an increase in ROS generation in the cytosol and the mitochondrial intermembrane space (IMS) during hypoxia. All of these results indicate that acute hypoxia generated more ROS in complex III and increased mitochondrial oxygen consumption, leading to less ATP production. In conclusion, acute hypoxia depresses the mitochondrial oxygen utilization efficiency by decreasing ATP production and increasing oxygen consumption as a result of the enhanced ROS generation at mitochondrial complex III.




Similar content being viewed by others
References
Ando T, Imamura H, Suzuki R, Aizaki H, Watanabe T, Wakita T, Suzuki T (2012) Visualization and measurement of ATP levels in living cells replicating hepatitis C virus genome RNA. PLoS Pathog 8:e1002561. https://doi.org/10.1371/journal.ppat.1002561
Chang H, Zhang L, Xu PT, Li Q, Sheng JJ, Wang YY, Chen Y, Zhang LN, Yu ZB (2011) Nuclear translocation of calpain-2 regulates propensity toward apoptosis in cardiomyocytes of tail-suspended rats. J Cell Biochem 112:571–580. https://doi.org/10.1002/jcb.22947
Di Cara F, Duca E, Dunbar DR, Cagney G, Heck MMS (2013) Invadolysin, a conserved lipid-droplet-associated metalloproteinase, is required for mitochondrial function in Drosophila. J Cell Sci 126:4769–4781 http://jcs.biologists.org/cgi/doi/4710.1242/jcs.133306
Divakaruni AS, Brand MD (2011) The regulation and physiology of mitochondrial proton leak. Physiology 26:192–205
Dooley CT, Dore TM, Hanson GT, Jackson WC, Remington SJ, Tsien RY (2004) Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J Biol Chem 279:22284–22293
Hanson GT, Aggeler R, Oglesbee D, Cannon M, Capaldi RA, Tsien RY, Remington SJ (2004) Investigating mitochondrial redox potential with redox-sensitive green fluorescent protein indicators. J Biol Chem 279:13044–13053
Imray C, Wright A, Subudhi A, Roach R (2010) Acute mountain sickness: pathophysiology, prevention, and treatment. Prog Cardiovasc Dis 52:467–484 https://linkinghub.elsevier.com/retrieve/pii/S0033062010000307
Kioka H, Kato H, Fujikawa M, Tsukamoto O, Suzuki T, Imamura H, Nakano A, Higo S, Yamazaki S, Matsuzaki T, Takafuji K, Asanuma H, Asakura M, Minamino T, Shintani Y, Yoshida M, Noji H, Kitakaze M, Komuro I, Asano Y, Takashima S (2014) Evaluation of intramitochondrial ATP levels identifies G0/G1 switch gene 2 as a positive regulator of oxidative phosphorylation. Proc Natl Acad Sci 111:273–278 http://www.pnas.org/cgi/doi/210.1073/pnas.1318547111
Levine BD (2002) Intermittent hypoxic training: fact and fancy. High Alt Med Biol 3:177–193
Maddocks ODK, Berkers CR, Mason SM, Zheng L, Blyth K, Gottlieb E, Vousden KH (2013) Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature 493:542–546 http://www.nature.com/articles/nature11743
Mailloux RJ, Harper M-E (2011) Uncoupling proteins and the control of mitochondrial reactive oxygen species production. Free Radic Biol Med 51:1106–1115
McElroy GS, Chandel NS (2017) Mitochondria control acute and chronic responses to hypoxia. Exp Cell Res 356:217–222 https://linkinghub.elsevier.com/retrieve/pii/S0014482717301556
Muza SR (2007) Military applications of hypoxic training for high-altitude operations. Med Sci Sports Exerc 39:1625–1631
Muza SR, Beidleman BA, Fulco CS (2010) Altitude preexposure recommendations for inducing acclimatization. High Alt Med Biol 11:87–92 http://www.liebertpub.com/doi/10.1089/ham.2010.1006
Nakano M, Imamura H, Nagai T, Noji H (2011) Ca <sup>2 + </sup> Regulation of mitochondrial ATP synthesis visualized at the single cell level. ACS Chem Biol 6:709–715 https://pubs.acs.org/doi/710.1021/cb100313n
Ponugoti B, Xu F, Zhang C, Tian C, Pacios S, Graves DT (2013) FOXO1 promotes wound healing through the up-regulation of TGF-β1 and prevention of oxidative stress. J Cell Biol 203:327–343 https://rupress.org/jcb/article/203/322/327/37572/FOXO37571-promotes-wound-healing-through-the
Scott GR (2011) Elevated performance: the unique physiology of birds that fly at high altitudes. J Exp Biol 214:2455–2462 http://jeb.biologists.org/cgi/doi/2410.1242/jeb.052548
Scott GR, Egginton S, Richards JG, Milsom WK (2009) Evolution of muscle phenotype for extreme high altitude flight in the bar-headed goose. Proc R Soc B Biol Sci 276:3645–3653 https://royalsocietypublishing.org/doi/3610.1098/rspb.2009.0947
Scott GR, Richards JG, Milsom WK (2009) Control of respiration in flight muscle from the high-altitude bar-headed goose and low-altitude birds. Am J Phys Regul Integr Comp Phys 297:R1066–R1074 https://www.physiology.org/doi/1010.1152/ajpregu.00241.02009
Scott GR, Schulte PM, Egginton S, Scott ALM, Richards JG, Milsom WK (2011) Molecular evolution of cytochrome c oxidase underlies high-altitude adaptation in the bar-headed goose. Mol Biol Evol 28:351–363 https://academic.oup.com/mbe/article-lookup/doi/310.1093/molbev/msq1205
Sun S-Z, Wei L, Wei D-B, Wang D-W, Ma B-Y (2013) Differences of glycolysis in skeletal muscle and lactate metabolism in liver between plateau zokor (Myospalax baileyi) and plateau pika (Ochotona curzoniae). Sheng Li Xue Bao 65:276–284
Vevea JD, Alessi Wolken DM, Swayne TC, White AB, Pon LA (2013) Ratiometric biosensors that measure mitochondrial redox state and ATP in living yeast cells. J Vis Exp:50633 %U http://www.jove.com/video/50633/ratiometric-biosensors-that-measure-mitochondrial-redox-state-atp
Waypa GB, Marks JD, Guzy R, Mungai PT, Schriewer J, Dokic D, Schumacker PT (2010) Hypoxia triggers subcellular compartmental redox signaling in vascular smooth muscle cells. Circ Res 106:526–535
Zhao R-Z, Jiang S, Ru N-Y, Jiao B, Yu Z-B (2019) Comparison of hypoxic effects induced by chemical and physical hypoxia on cardiomyocytes. Can J Physiol Pharmacol 97:980–988 http://www.nrcresearchpress.com/doi/910.1139/cjpp-2019-0092
Zhao RZ, Jiang S, Zhang L, Yu ZB (2019) Mitochondrial electron transport chain, ROS generation and uncoupling (Review) %U http://www.spandidos-publications.com/10.3892/ijmm.2019.4188. Int J Mol Med
Zielonka J, Kalyanaraman B (2018) Small-molecule luminescent probes for the detection of cellular oxidizing and nitrating species. Free Radic Biol Med 128:3–22 https://linkinghub.elsevier.com/retrieve/pii/S0891584918301357
Acknowledgments
The authors sincerely thank Professor Imamura (Kyoto University) for giving us the pcDNA-mitGo-ATeam2.
Funding
This work was supported by the National Natural Science Foundation of China grant No. 81571844.
Author information
Authors and Affiliations
Contributions
Every author made a valuable contribution to the preparation of this manuscript. ZBY and YYW conceived and designed the study. RZZ, XBW, SJ, and NYR performed the experiments. RZZ and XBW analyzed the data. RZZ and BJ produced the figures. RZZ wrote the manuscript. ZBY and YYW critically revised the manuscript. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
All animal experiments were performed in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals and were approved by the Ethics Committee for the animal care and use of Fourth Military Medical University.
Conflict of interest
The authors declare that they have no competing interests.
Informed consent
All authors declared their informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, RZ., Wang, XB., Jiang, S. et al. Elevated ROS depress mitochondrial oxygen utilization efficiency in cardiomyocytes during acute hypoxia. Pflugers Arch - Eur J Physiol 472, 1619–1630 (2020). https://doi.org/10.1007/s00424-020-02463-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-020-02463-5