Abstract
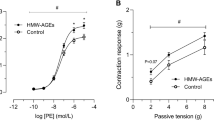
We investigated the direct effects of prolonged exposure to advanced glycation end-products (AGEs) on noradrenaline-induced contraction of rat carotid artery smooth muscle. Noradrenaline-induced contraction of endothelium-denuded carotid artery rings was suppressed by AGE-bovine serum albumin (AGE-BSA) pretreatment (0.01 and 0.1 mg/mL for 23 ± 1 h) compared with vehicle pretreatment (control), whereas isotonic-K+-induced contraction was not significantly altered by AGE-BSA pretreatment. This reduction in noradrenaline-induced contraction by AGE-BSA (0.1 mg/mL) was reversed by iberiotoxin, an inhibitor of large-conductance calcium-activated potassium (BKCa) channels, but not by inhibitors of other K channels [4-AP (Kv inhibitor), TRAM-34 (IKCa inhibitor), or glibenclamide (KATP inhibitor)]. Acute incubation of carotid arterial rings with H2O2 had also reduced noradrenaline-induced contraction in control arteries, but it had no effect on noradrenaline-induced contraction in AGE-BSA-pretreated arteries. Alternatively, acute incubation with the H2O2 scavenger catalase increased noradrenaline-induced contraction of AGE-BSA-pretreated arteries but had no effect on noradrenaline-induced contraction of control arteries. Noradrenaline-induced contraction in the presence of H2O2 was increased by co-treatment with iberiotoxin. The AGE-BSA-mediated suppression of noradrenaline-induced contraction was prevented by the organic cation transporter 3 (OCT3) inhibitor corticosterone, whereas the expression of OCT3 protein was similar between control and AGE-BSA-treated endothelium-denuded carotid arteries. These findings suggest that noradrenaline-induced arterial contraction is reduced by prolonged AGE-BSA exposure due to activation of BKCa channels via H2O2 generation and increased OCT3-mediated noradrenaline transport activity.






Similar content being viewed by others
References
Allahverdian S, Chaabane C, Boukais K, Francis GA, Bochaton-Piallat ML (2018) Smooth muscle cell fate and plasticity in atherosclerosis. Cardiovasc Res 114:540–550
Ando M, Matsumoto T, Taguchi K, Kobayashi T (2017) Poly (I:C) impairs NO donor-induced relaxation by overexposure to NO via the NF-kappa B/iNOS pathway in rat superior mesenteric arteries. Free Radic Biol Med 112:553–566
Ayala-Lopez N, Jackson WF, Burnett R, Wilson JN, Thompson JM, Watts SW (2015) Organic cation transporter 3 contributes to norepinephrine uptake into perivascular adipose tissue. Am J Physiol Heart Circ Physiol 309:H1904–H1914
Barlow RS, White RE (1998) Hydrogen peroxide relaxes porcine coronary arteries by stimulating BKCa channel activity. Am J Phys 275:H1283–H1289
Barlow RS, El-Mowafy AM, White RE (2000) H(2)O(2) opens BK(Ca) channels via the PLA(2)-arachidonic acid signaling cascade in coronary artery smooth muscle. Am J Physiol Heart Circ Physiol 279:H475–H483
Bartho LA, Holland OJ, Moritz KM, Perkins AV, Cuffe JSM (2019) Maternal corticosterone in the mouse alters oxidative stress markers, antioxidant function and mitochondrial content in placentas of female fetuses. J Physiol 597:3053–3067
Burnstock G (2009) Autonomic neurotransmission: 60 years since sir Hanry Dale. Annu Rev Pharmacol Toxicol 49:1–30
Cato AC, Nestl A, Mink S (2002) Rapid actions of steroid receptors in cellular signaling pathway. Sci STKE 2002:RE9
Ellis A, Pannirselvan M, Anderson TJ, Triggle CR (2003) Catalase has negligible inhibitory effects on endothelium-dependent relaxations in mouse isolated aorta and small mesenteric artery. Br J Pharmacol 140:1193–1200
Fishman SL, Sonmez H, Basman C, Singh V, Poretsky L (2018) The role of advanced glycation end-products in the development of coronary artery disease in patients with and without diabetes mellitus: a review. Mol Med 24:59
Fleming TH, Humpert PM, Nawroth PP, Bierhaus A (2011) Reactive metabolites and AGE/RAGE-mediated cellular dysfunction affect the aging process: a mini-review. Gerontology 57:435–443
Frismantiene A, Philippova M, Erne P, Resink TJ (2018) Smooth muscle cell-driven vascular diseases and molecular mechanisms of VSMC plasticity. Cell Signal 52:48–64
Fujimoto S, Mori M, Tsushima H (2003) Mechanisms underlying the hydrogen-peroxide-induced, endothelium-independent relaxation of the norepinephrine-contraction in guinea-pig aorta. Eur J Pharmacol 459:65–73
Goldin A, Beckman JA, Schmidt AM, Creager MA (2006) Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation 114:597–605
Grootaert MOJ, Moulis M, Roth L, Martinet W, Vindis C, Bennett MR, De Meyer GRY (2018) Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc Res 114:622–634
Henrion D, Laher I (1993) Potentiation of norepinephrine-induced contractions by endothelin-1 in the rabbit aorta. Hypertension 22:78–83
Henrion D, Laher I, Laporte R, Bevan JA (1992) Further evidence from an elastic artery that angiotensin II amplifies noradrenaline-induced contraction through activation of protein kinase C. Eur J Pharmacol 224:13–20
Horvath G, Torbati A, Conner GE, Salathe M, Wanner A (2001) Steroid sensitivity of norepinephrine uptake by human bronchial arterial and rabbit aortic smooth muscle cells. Am J Respir Cell Mol Biol 25:500–506
Horvath G, Sutto Z, Torbati A, Conner GE, Salathe M, Wanner A (2003) Norepinephrine transport by the extraneuronal monoamine transporter in human bronchial arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 285:L829–L837
Jackson WF (2017) Potassium channels in regulation of vascular smooth muscle contraction and growth. Adv Pharmacol 78:89–144
Johnstone WW 3rd, Honeycutt JL, Deck CA, Borski RJ (2019) Nongenomic glucocorticoid effects and their mechanisms of action in vertebrates. Int Rev Cell Mol Biol 346:51–96
Khammy MM, Kim S, Bentzen BH, Lee S, Choi I, Aalkjaer C, Jepps TA (2018) 4-Aminopyridine: a pan voltage-gated potassium channel inhibitor that enhances Kv7.4 currents and inhibits noradrenaline-mediated contraction of rat mesenteric small arteries. Br J Pharmacol 175:501–516
Kobayashi T, Kamata K (2002) Modulation by hydrogen peroxide of noradrenaline-induced contraction in aorta from streptozotocin-induced diabetic rat. Eur J Pharmacol 441:83–89
Kobayashi T, Kaneda A, Kamata K (2003) Possible involvement of IGF-1 receptor and IGF-binding protein in insulin-induced enhancement of noradrenaline response in diabetic rat aorta. Br J Pharmacol 140:285–294
Kobayashi T, Matsumoto T, Kamata K (2005) IFG-I-induced enhancement of contractile response in organ-cultured aortae from diabetic rats is mediated by sustained thromboxane A2 release from endothelial cells. J Endocrinol 186:367–376
Koepsell H, Lips K, Volk C (2007) Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res 24:1227–1251
Lan KC, Chiu CY, Kao CW, Huang KH, Wang CC, Huang KT, Tsai KS, Sheu ML, Liu SH (2015) Advanced glycation end-products induce apoptosis in pancreatic islet endothelial cells via NF-kB-activated cyclooxygenase-2/prostaglandin E2 up-regulation. PLoS One 10:e0124418
Laporte R, DeRoth L (1997) Modulation of the effects of norepinephrine uptake inhibitors on the norepinephrine-induced contractile response of the porcine uterine artery during early pregnancy. Can J Vet Res 61:214–220
Li SS, Wu Y, Jin X, Jiang C (2015) The SUR2B subunit of rat vascular KATP channel is targeted by miR-9a-3p induced by prolonged exposure to methylglyoxal. Am J Physiol Cell Physiol 308:C139–C145
Matsumoto T, Kobayashi T, Ishida K, Hirasawa Y, Morita H, Honda T, Kamata K (2010) Vasodilator effect of Cassiarin A, a novel antiplasmodial alkaloid from Cassia siamea, in rat isolated mesenteric artery. Biol Pharm Bull 33:844–848
Matsumoto T, Szasz T, Tostes RC, Webb RC (2012) Impaired β-adrenoceptor-induced relaxation in small mesenteric arteries from DOCA-salt hypertensive rats is due to reduced K(Ca) channel activity. Pharmacol Res 65:537–545
Matsumoto T, Watanabe S, Iguchi M, Ando M, Oda M, Nagata M, Yamada K, Taguchi K, Kobayashi T (2016) Mechanisms underlying enhanced noradrenaline-induced femoral arterial contractions of spontaneously hypertensive rats: involvement of endothelium-derived factors and cyclooxygenase-derived prostanoids. Biol Pharm Bull 39:384–393
Matsumoto T, Kojima M, Takayanagi K, Katome T, Taguchi K, Kobayashi T (2019) Amplification of the COX/TXS/TP receptor pathway enhances uridine diphosphate-induced contraction by advanced glycation end products in rat carotid arteries. Pflugers Arch 471:1505–1517
Mukohda M, Yamawaki H, Nomura H, Okada M, Hara Y (2009) Methylglyoxal inhibits smooth muscle contraction in isolated blood vessels. J Pharmacol Sci 109:305–310
Mukohda M, Morita T, Okada M, Hara Y, Yamawaki H (2012) Long-term methylglyoxal treatment impairs smooth muscle contractility in organ-cultured rat mesenteric artery. Pharmacol Res 65:91–99
Nah SS, Choi IY, Lee CK, Oh JS, Kim YG, Moon HB, Yoo B (2008) Effects of advanced glycation end products on the expression of COX-2, PGE2, NO in human osteroarthritic chondrocytes. Rheumatology 47:425–431
Ohkubo H, Chiba S (1989) Vascular response of ophthalmic arteries to exogenous and endogenous norepinephrine. Exp Eye Res 48:539–547
Ozaki H, Karaki H (2002) Organ culture as a useful method for studying the biology of blood vessels and other smooth muscle tissues. Jpn J Pharmacol 89:93–100
Santiago E, Martinez MP, Climent B, Munoz M, Briones AM, Salaices M, Garcia-Sacristan A, Rivera L, Prieto D (2016) Augmented oxidative stress and preserved vasoconstriction induced by hydrogen peroxide in coronary arteries in obesity: role of COX-2. Br J Pharmacol 173:3176–3195
Saxton SN, Ryding KE, Aldous RG, Withers SB, Ohanian J, Heagerty AM (2018) Role of sympathetic nerves and adipocyte catecholamine uptake in the vasorelaxant function of perivascular adipose tissue. Arterioscler Thromb Vasc Biol 38:880–891
Shi L, Liu B, Li N, Xue Z, Liu X (2013) Aerobic exercise increase BK(Ca) channel contribution to regulation of mesenteric arterial tone by upregulating β1-subunit. Exp Physiol 98:326–336
Simard E, Sollradl T, Maltais JS, Boucher J, D’Orleans-Juste P, Grandbois M (2015) Receptor for advanced glycation end-products signaling interferes with the vascular smooth muscle cell contractile phenotype and function. PLoS One 10:e0128881
Stirban A, Gawlowski T, Roden M (2013) Vascular effects of advanced glycation endproducts: clinical effects and molecular mechanisms. Mol Metab 3:94–108
Su J, Lucchesi PA, Gonzalez-Villalobos RA, Palen DI, Rezk BM, Suzuki Y, Boulares HA, Matrougui K (2008) Role of advanced glycation end products with oxidative stress in resistance artery dysfunction in type 2 diabetic mice. Arterioscler Thromb Vasc Biol 28:1432–1438
Su W, Li W, Chen H, Liu H, Huang H, Li H (2015) Advanced glycation end products impair voltage-gated K+ channels-mediated coronary vasodilation in diabetic rats. PLoS One 10:e014865
Teoh H, Quan A, Leung SW, Man RY (2000) Differential effects of 17beta-estradiol and testosterone on the contractile responses of porcine coronary arteries. Br J Pharmacol 129:1301–1308
Valverde MA, Rojas P, Amigo J, Comsmelli D, Orio P, Bahamonde MI, Mann GE, Vergara C, Latorre R (1999) Acute activation of maxi-K channels (hSlo) by estradiol binding to the β-subunits. Science 285:1929–1931
Vila E, Vivas NM, Tabernero A, Giraldo J, Arribas SM (1997) Alpha 1-adrenoceptor vasoconstriction in the tail artery during ageing. Br J Pharmacol 121:1017–1023
Wahlestedt C, Edvinsson L, Ekblad E, Hakanson R (1985) Neuropeptide Y potentiates noradrenaline-evoked vasoconstriction: mode of action. J Pharmacol Exp Ther 234:735–741
Wang X, Chang T, Jiang B, Desai K, Wu L (2007) Attenuation of hypertension development by aminoguanidine in spontaneously hypertensive rats: role of methylglyoxal. Am J Hypertens 20:629–636
Watanabe S, Matsumoto T, Ando M, Adachi T, Kobayashi S, Iguchi M, Takeuchi M, Taguchi K, Kobayashi T (2016) Multiple activation mechanisms of serotonin-mediated contraction in the carotid arteries obtained from spontaneously hypertensive rats. Pflugers Arch 468:1271–1282
Watanabe S, Matsumoto T, Oda M, Yamada K, Takagi J, Taguchi K, Kobayashi T (2016) Insulin augments serotonin-induced contraction via activation of the IR/PI3K/PDK1 pathway in the rat carotid artery. Pflugers Arch 468:667–677
Wautier JL, Schmidt AM (2004) Protein glycation: a firm link to endothelial cell dysfunction. Circ Res 95:233–238
Wilkenfeld SR, Lin C, Frigo DE (2018) Communication between genomic and non-genomic signaling events coordinate steroid hormone actions. Steroids 133:2–7
Xu B, Ji Y, Yao K, Cao YX, Ferro A (2005) Inhibition of human endothelial cell nitric oxide synthase by advanced glycation end-products but not glucose: relevance to diabetes. Clin Sci 109:439–446
Yamagishi S, Nakamura N, Suematsu M, Kaseda K, Matsui T (2015) Advanced glycation end products: a molecular target for vascular complications in diabetes. Mol Med 21:S32–S40
Yamawaki H, Sato K, Hori M, Ozaki H, Nakamura S, Nakayama H, Doi K, Karaki H (1999) Impairment of EDR by a long-term PDGF treatment in organ-cultured rabbit mesenteric artery. Am J Physiol 277:H318–H323
Yin QF, Xiong Y (2005) Pravastatin restores DDAH activity and endothelium-dependent relaxation of rat aorta after exposure to glycated protein. J Cardiovasc Pharmacol 45:525–532
Zhao LM, Su XL, Wang Y, Li GR, Deng XL (2013) KCa3.1 channels mediate the increase of cell migration and proliferation by advanced glycation endproducts in cultured rat vascular smooth muscle cell. Lab Investig 93:159–167
Zhao LM, Wang Y, Ma XZ, Wang NP, Deng XL (2014) Advanced glycation end products impair K(Ca)3.1- and K(Ca)2.3-mediated vasodilatation via oxidative stress in rat mesenteric arteries. Pflugers Arch 466:307–317
Zhu Y, Ma WQ, Han XQ, Wang Y, Wang X, Liu NF (2018) Advanced glycation end products accelerate calcification in VSMCs through HIF-1α/PDK4 activation and suppress glucose metabolism. Sci Rep 8:13730
Acknowledgments
We would like to thank Tomoki Katome, Yuri Asano, Akari Ishibiki, Saya Imamura, Myu Kozakai, Kana Saegusa, Kanako Takimoto, Takeru Toda, Yuka Hayashida, Taiga Yoshida, Marina Ito, Yurika Ezaki, Amane Kurakata, Yuzuki Sato, Tamayo Hashimoto, Yurina Mae, and Hiyori Yokoyama for the excellent technical assistance. We also thank Enago (www.enago.jp) for the English language review.
Funding
This work was supported in part by grants JSPS KAKENHI grant numbers JP18K06861 (to Takayuki Matsumoto), JP17K08318 (to Kumiko Taguchi), and JP18K06974 (to Tsuneo Kobayashi).
Author information
Authors and Affiliations
Contributions
T.M. and T.K. conceived and designed the research; T.M., K.T., and M.K. performed the experiments; T.M., K.T., M.K., T.K, and K.T. analyzed the data; T.M., K.T., M.K., T.K., K.T., and T.K. interpreted the results; T.M. prepared the figures; T.M. drafted the manuscript; T.M. and T.K. edited and revised the manuscript; T.M., K.T., M.K., T.K., K.T., and T.K. approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Matsumoto, T., Takayanagi, K., Kojima, M. et al. Mechanisms underlying suppression of noradrenaline-induced contraction by prolonged treatment with advanced glycation end-products in organ-cultured rat carotid artery. Pflugers Arch - Eur J Physiol 472, 355–366 (2020). https://doi.org/10.1007/s00424-020-02349-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-020-02349-6