Abstract
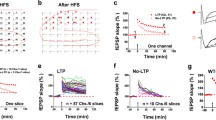
In a previous study, we pointed out that the neurotoxic action evoked by methylmercury (MeHg), a potent environmental pollutant responsible for fatal food poisoning, is associated with alterations of cellular excitability by irreversible blockade of sodium and calcium currents. Here, we investigated the MeHg effects on synaptic transmission and neuronal plasticity using extracellular field recording in CA1 area of rat hippocampal slices. MeHg caused a fast and drastic depression of evoked field excitatory postsynaptic potentials (fEPSPs) in a concentration-dependent manner with an IC50 of 25.7 μM. This depression was partially caused by the irreversible reduction of axon recruitment deduced from the decrement of the fiber volley (FV) amplitude. Nevertheless, this MeHg-induced synaptic depression represents a true reduction of synaptic efficacy, as judged by input/output curves. In addition, a reduction on presynaptic release of glutamate was detected with the paradigm of paired-pulse facilitation during MeHg application. Moreover, MeHg also reduced population spike (PS) ampxlitude, and this effect was more prominent when the PS was evoked by ortodromic stimulation than by antidromic stimulation. Interestingly, despite these strong effects of MeHg on synaptic transmission and excitability, this compound did not modify the induction of long-term synaptic potentiation (LTP). The effects described here for MeHg were irreversible or very slowly reversible after drug wash-out. In summary, the blockade of sodium and calcium channels by MeHg affects synaptic transmission and cellular excitability but not synaptic plasticity.






Similar content being viewed by others
References
Andersen P (1960) Interhippocampal impulses. 2. Apical dendritic activation of CAL neurons. AFOSR TN United States Air Force Off Sci Res 59:998–991
Aschner M, Aschner JL (1990) Mercury neurotoxicity: mechanisms of blood-brain barrier transport. Neurosci Biobehav Rev 14(2):169–176
Atchison WD (2005) Is chemical neurotransmission altered specifically during methylmercury-induced cerebellar dysfunction? Trends Pharmacol Sci 26(11):549–557
Atchison WD, Hare MF (1994) Mechanisms of methylmercury-induced neurotoxicity. FASEB J 8(9):622–629
Atchison WD, Narahashi T (1982) Methylmercury-induced depression of neuromuscular transmission in the rat. Neurotoxicology 3(3):37–50
Bakir F, Damluji SF, Amin-Zaki L, Murtadha M, Khalidi A, al-Rawi NY, Tikriti S, Dhahir HI, Clarkson TW, Smith JC, Doherty RA (1973) Methylmercury poisoning in Iraq. Science 181(4096):230–241
Baldelli P, Forni PE, Carbone E (2000) BDNF, NT-3 and NGF induce distinct new Ca2+ channel synthesis in developing hippocampal neurons. Eur J Neurosci 12(11):4017–4032
Bliss T, Lømo T (1973) Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol 232:331–356
Burgoyne RD, Alvarez de Toledo G (2000) Fusion proteins and fusion pores. Workshop: regulated exocytosis and the vesicle cycle. EMBO Rep 1(4):304–307
Carocci A1, Rovito N, Sinicropi MS, Genchi G (2014) Mercury toxicity and neurodegenerative effects. Rev Environ Contam Toxicol 229:1–18
Eyl TB (1971) Organic-mercury food poisoning. N Engl J Med 284(13):706–709
Falluel-Morel A, Lin L, Sokolowski K, McCandlish E, Buckley B, DiCicco-Bloom E (2012) N-Acetyl cysteine treatment reduces mercury-induced neurotoxicity in the developing rat hippocampus. J Neurosci Res 90(4):743–750
Fountain SB, Rowan JD (2000) Effects of sequential exposure to multiple concentrations of methylmercury in the rat hippocampal slice. Ecotox Environ Safe 47(2):130–136
Franco JL, Posser T, Dunkley PR, Dickson PW, Mattos JJ, Martins R, Bainy ACD, Marques MR, Dafre AL, Farina M (2009) Methylmercury neurotoxicity is associated with inhibition of the antioxidant enzyme glutathione peroxidase. Free Radic Biol Med 47(4):449–457
Friberg L, Mottet NK (1989) Accumulation of methylmercury and inorganic mercury in the brain. Biol Trace Elem Res 21:201–206
Fuentes-Antras J, Osorio-Martinez E, Ramirez-Torres M, Colmena I, Fernandez-Morales JC, Hernandez-Guijo JM (2013) Methylmercury decreases cellular excitability by a direct blockade of sodium and calcium channels in bovine chromaffin cells: an integrative study. Pflugers Arch 465(12):1727–1740
Hajela RK, Peng SQ, Atchison WD (2003) Comparative effects of methylmercury and Hg(2+) on human neuronal N- and R-type high-voltage activated calcium channels transiently expressed in human embryonic kidney 293 cells. J Pharmacol Exp Ther 306(3):1129–1136
Hess G, Kuhnt U (1992) Presynaptic calcium transients evoked by paired-pulse stimulation in the hippocampal slice. Neuroreport 3(4):361–364
Hodgkin AL, Huxley AF (1952) A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol 117(4):500–544
Katz B, Miledi R (1968) The role of calcium in neuromuscular facilitation. J Physiol 195(2):481–492
Leonhardt R, Haas H, Büsselberg D (1996) Methyl mercury reduces voltage-activated currents of rat dorsal root ganglion neurons. Naunyn Schmiedeberg’s Arch Pharmacol 354(4):532–538
Leonhardt R, Pekel M, Platt B, Haas HL, Busselberg D (1996) Voltage-activated calcium channel currents of rat DRG neurons are reduced by mercuric chloride (HgCl2) and methylmercury (CH3HgCl). Neurotoxicology 17(1):85–92
Manabe T, Wyllie DJ, Perkel DJ, Nicoll RA (1993) Modulation of synaptic transmission and long-term potentiation: effects on paired pulse facilitation and EPSC variance in the CA1 region of the hippocampus. J Neurophysiol 70(4):1451–1459
Mancini JD, Autio DM, Atchison WD (2009) Continuous exposure to low concentrations of methylmercury impairs cerebellar granule cell migration in organotypic slice culture. Neurotoxicology 30(2):203–208
Merad-Boudia M, Nicole A, Santiard-Baron D, Saillé C, Ceballos-Picot I (1998) Mitochondrial impairment as an early event in the process of apoptosis induced by glutathione depletion in neuronal cells: relevance to Parkinson’s disease. Biochem Pharmacol 56(5):645–655
Myers GJ, Davidson PW (1998) Prenatal methylmercury exposure and children: neurologic, developmental, and behavioral research. Environ Health Perspect 106(Suppl 3):841–847
Ochi T (2002) Methylmercury, but not inorganic mercury, causes abnormality of centrosome integrity (multiple foci of gamma-tubulin), multipolar spindles and multinucleated cells without microtubule disruption in cultured Chinese hamster V79 cells. Toxicology 175(1–3):111–121
Peng S, Hajela RK, Atchison WD (2002) Effects of methylmercury on human neuronal L-type calcium channels transiently expressed in human embryonic kidney cells (HEK-293). J Pharmacol Exp Ther 302(2):424–432
Protti DA, Uchitel OD (1993) Transmitter release and presynaptic Ca2+ currents blocked by the spider toxin omega-Aga-IVA. Neuroreport 5(3):333–336
Quandt FN, Kato E, Narahashi T (1982) Effects of methylmercury on electrical responses of neuroblastoma cells. Neurotoxicology 3(4):205–220
Raastad M, Shepherd GM (2003) Single-axon action potentials in the rat hippocampal cortex. J Physiol 548(Pt 3):745–752
Rao MV, Purohit A, Patel T (2010) Melatonin protection on mercury-exerted brain toxicity in the rat. Drug Chem Toxicol 33(2):209–216
Roda E, Coccini T, Acerbi D, Castoldi A, Bernocchi G, Manzo L (2008) Cerebellum cholinergic muscarinic receptor (subtype-2 and -3) and cytoarchitecture after developmental exposure to methylmercury: an immunohistochemical study in rat. J Chem Neuroanat 35(3):285–294
Sakaue M, Mori N, Makita M, Fujishima K, Hara S, Arishima K, Yamamoto M (2009) Acceleration of methylmercury-induced cell death of rat cerebellar neurons by brain-derived neurotrophic factor in vitro. Brain Res 1273:155–162
Sarafian T, Verity MA (1991) Oxidative mechanisms underlying methyl mercury neurotoxicity. Int J Dev Neurosci 9(2):147–153
Shafer TJ, Atchison WD (1992) Effects of methylmercury on perineurial Na+ and Ca2+-dependent potentials at neuromuscular junctions of the mouse. Brain Res 595(2):215–219
Sirois JE, Atchison WD (2000) Methylmercury affects multiple subtypes of calcium channels in rat cerebellar granule cells. Toxicol Appl Pharmacol 167(1):1–11
Szucs A, Angiello C, Salanki J, Carpenter DO (1997) Effects of inorganic mercury and methylmercury on the ionic currents of cultured rat hippocampal neurons. Cell Mol Neurobiol 17(3):273–288
Takahashi T, Momiyama A (1993) Different types of calcium channels mediate central synaptic transmission. Nature 366(6451):156–158
Takeuchi T (1982) Pathology of Minamata disease. With special reference to its pathogenesis. Acta Pathol Jpn 32(Suppl 1):73–99
Yuan Y, Atchison WD (1993) Disruption by methylmercury of membrane excitability and synaptic transmission of CA1 neurons in hippocampal slices of the rat. Toxicol Appl Pharmacol 120(2):203–215
Yuan Y, Atchison WD (1995) Methylmercury acts at multiple sites to block hippocampal synaptic transmission. J Pharmacol Exp Ther 275(3):1308–1316
Yuan Y, Atchison WD (1997) Action of methylmercury on GABA(A) receptor-mediated inhibitory synaptic transmission is primarily responsible for its early stimulatory effects on hippocampal CA1 excitatory synaptic transmission. J Pharmacol Exp Ther 282(1):64–73
Yuan Y, Atchison WD (2007) Methylmercury-induced increase of intracellular Ca2+ increases spontaneous synaptic current frequency in rat cerebellar slices. Mol Pharmacol 71(4):1109–1121
Acknowledgements
G.J. is a fellow of MEC. E.A. is a fellow of FTH. The authors gratefully acknowledge the technical assistance of José Barbado.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gutiérrez, J., Baraibar, A.M., Albiñana, E. et al. Methylmercury reduces synaptic transmission and neuronal excitability in rat hippocampal slices. Pflugers Arch - Eur J Physiol 470, 1221–1230 (2018). https://doi.org/10.1007/s00424-018-2144-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-018-2144-x