Abstract
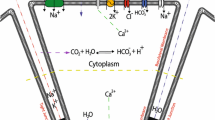
The plasma membrane of parotid acinar cells is functionally divided into apical and basolateral regions. According to the current model, fluid secretion is driven by transepithelial ion gradient, which facilitates water movement by osmosis into the acinar lumen from the interstitium. The osmotic gradient is created by the apical Cl− efflux and the subsequent paracellular Na+ transport. In this model, the Na+-K+ pump is located exclusively in the basolateral membrane and has essential role in salivary secretion, since the driving force for Cl− transport via basolateral Na+–K+–2Cl− cotransport is generated by the Na+-K+ pump. In addition, the continuous electrochemical gradient for Cl− flow during acinar cell stimulation is maintained by the basolateral K+ efflux. However, using a combination of single-cell electrophysiology and Ca2+-imaging, we demonstrate that photolysis of Ca2+ close to the apical membrane of parotid acinar cells triggered significant K+ current, indicating that a substantial amount of K+ is secreted into the lumen during stimulation. Nevertheless, the K+ content of the primary saliva is relatively low, suggesting that K+ might be reabsorbed through the apical membrane. Therefore, we investigated the localization of Na+-K+ pumps in acinar cells. We show that the pumps appear evenly distributed throughout the whole plasma membrane, including the apical pole of the cell. Based on these results, a new mathematical model of salivary fluid secretion is presented, where the pump reabsorbs K+ from and secretes Na+ to the lumen, which can partially supplement the paracellular Na+ pathway.






Similar content being viewed by others
References
Almassy J, Yule DI (2013) Analyzing ca(2+) dynamics in intact epithelial cells using spatially limited flash photolysis. Cold Spring Harb Protoc 2013(1). https://doi.org/10.1101/pdb.prot072777
Almassy J, Yule DI (2013) Investigating ion channel distribution using a combination of spatially limited photolysis, Ca(2+) imaging, and patch clamp recording. Cold Spring Harb Protoc 2013(1). https://doi.org/10.1101/pdb.prot072769
Almassy J, Yule DI (2013) Studying the activation of epithelial ion channels using global whole-field photolysis. Cold Spring Harb Protoc 2013(1). https://doi.org/10.1101/pdb.prot072751
Almassy J, Yule DI (2013) Photolysis of caged compounds: studying Ca(2+) signaling and activation of Ca(2+)-dependent ion channels. Cold Spring Harb Protoc 2013(1). https://doi.org/10.1101/pdb.top066076
Almassy J, Won JH, Begenisich TB, Yule DI (2012) Apical Ca2+-activated potassium channels in mouse parotid acinar cells. J Gen Physiol 139(2):121–133. https://doi.org/10.1085/jgp.201110718
Bundgaard M, Moller M, Poulsen JH (1977) Localization of sodium pump sites in cat salivary glands. J Physiol 273:339–353
Conteas CN, McDonough AA, Kozlowski TR, Hensley CB, Wood RL, Mircheff AK (1986) Mapping subcellular distribution of Na+-K+-ATPase in rat parotid gland. Am J Phys 250:430–441
Evans RL, Park K, Turner RJ, Watson GE, Nguyen HV, Dennett MR, Hand AR, Flagella M, Shull GE, Melvin JE (2000) Severe impairment of salivation in Na+/K+/2Cl− cotransporter (NKCC1)-deficient mice. J Biol Chem 275(35):26720–26726. https://doi.org/10.1074/jbc.M003753200
Garrett JR, Ekström J, Anderson LC (eds) (1998) Glandular mechanisms of salivary secretion. Front Oral Biol Basel, Karger vol 10:55–72
Garrett JR, Winston DC, Proctor GB, Schulte BA (1992) Na,K-ATPase in resting and stimulated submandibular salivary glands in cats, studied by means of ouabain-sensitive, K(+)-dependent p-nitrophenylphosphatase activity. Arch Oral Biol 37(9):711–716. https://doi.org/10.1016/0003-9969(92)90077-L
Iwano T, Akayama M, Yamamoto A, Omori K, Kumazawa T, Tashiro Y (1987) Quantitative immunoelectron microscopic localization of (Na+,K+)ATPase in rat parotid gland. J Histochem Cytochem 35(8):871–879. https://doi.org/10.1177/35.8.3036942
Larina O, Thorn P (2005) Ca2+ dynamics in salivary acinar cells: distinct morphology of the acinar lumen underlies near-synchronous global Ca2+ responses. J Cell Sci 118(18):4131–4139. https://doi.org/10.1242/jcs.02533
Mangos JA, McSherry N, Irwin K, Hong R (1973) Handling of water and electrolytes by rabbit parotid and submaxillary glands. Am J Phys 225:450–455
Mangos J, McSherry N, Nousia-Arvanitakis S, Irwin K (1973) Secretion and transductal fluxes of ions in exocrine glands of the mouse. Am J Phys 225:18–24
Martinez JR, Cassity N (1983) Effect of transport inhibitors on secretion by perfused rat submandibular gland. Am J Phys 245:711–716
Maruyama Y, Gallacher DV, Petersen OH (1983) Voltage and Ca2+-activated K+ channel in baso-lateral acinar cell membranes of mammalian salivary glands. Nature 302(5911):827–829. https://doi.org/10.1038/302827a0
Nakagaki I, Goto T, Sasaki S, Imai Y (1978) Histochemical and cytochemical localization of (Na+-K+)-activated adenosine triphosphatase in the acini of dog submandibular glands. J Histochem Cytochem 26(10):835–845. https://doi.org/10.1177/26.10.214493
Novak I, Young JA (1986) Two independent anion transport systems in rabbit mandibular salivary glands. Pflugers Arch 407(6):649–656. https://doi.org/10.1007/BF00582647
Palk L, Sneyd J, Shuttleworth TJ, Yule DI, Crampin EJ (2010) A dynamic model of saliva secretion. J Theor Biol 266(4):625–640. https://doi.org/10.1016/j.jtbi.2010.06.027
Park MK, Lomax RB, Tepikin AV, Petersen OH (2001) Local uncaging of caged Ca(2+) reveals distribution of Ca(2+)-activated Cl(-) channels in pancreatic acinar cells. Proc Natl Acad Sci U S A 98(19):10948–10953. https://doi.org/10.1073/pnas.181353798
Petersen OH, Poulsen JH (1967) Inhibition of salivary secretion and secretary potentials by g-strophantin, dinitrophenol and cyanide. Acta Physiol Scand 71(2-3):194–202. https://doi.org/10.1111/j.1748-1716.1967.tb03725.x
Petersen OH (1971) Oral physiology: proceedings of the international symposium held in Wenner-Gren center (edited by: Nils Emmelin, Yngve Zotterman) Pergamon Press Ltd. 21-31
Poulsen JH (1974) Acetylcholine-induced transport of Na+ and K+ in the perfused cat submandibular gland. Pflugers Arch 349(3):215–220. https://doi.org/10.1007/BF00592449
Poulsen JH (1974) Effects of ouabain on two types of active cation transport in the cat submandibular gland. In: Thorn NA, Petersen OH (eds) Secretory mechanisms of exocrine glands. Munksgaard, Copenhagen, pp 570–581
Poulsen JH, Bundgaard M (1994) Quantitative estimation of the area of luminal and basolateral membranes of rat parotid acinar cells: some physiological applications. Pflugers Arch 429(2):240–244. https://doi.org/10.1007/BF00374318
Romanenko V, Catalán MA, Brown DA, Putzier I, Hartzell HC, Marmorstein AD, Gonzalez-Begne M, Rock JR, Harfe BD, Melvin JE (2010) Tmem16A encodes the Ca2+-activated Cl- channel in mouse submandibular salivary gland acinar cells. J Biol Chem 285(17):12990–13001. https://doi.org/10.1074/jbc.M109.068544
Romanenko V, Nakamoto T, Srivastava A, Melvin JE, Begenisich T (2006) Molecular identification and physiological roles of parotid acinar cell maxi-K channels. J Biol Chem 281(38):27964–27972. https://doi.org/10.1074/jbc.M603871200
Romanenko V, Thompson J, Begenisich T (2010) Ca2+-activated K channels in parotid acinar cells: the functional basis for the hyperpolarized activation of BK channels. Channels (Austin) 4:278–288
Silva P, Stoff J, Field M, Fine L, Forrest JN, Epstein FH (1977) Mechanism of active chloride secretion by shark rectal gland: role of Na-K-ATPase in chloride transport. Am J Phys 233:298–306
Smith NP, Crampin EJ (2004) Development of models of active ion transport for whole-cell modelling: cardiac sodium-potassium pump as a case study. Prog Biophys Mol Biol 85(2-3):387–405. https://doi.org/10.1016/j.pbiomolbio.2004.01.010
Takahata T, Hayashi M, Ishikawa T (2003) SK4/IK1-like channels mediate TEA-insensitive, Ca2+-activated K+ currents in bovine parotid acinar cells. Am J Physiol Cell Physiol 284:127–144
Thompson J, Begenisich T (2006) Membrane-delimited inhibition of maxi-K channel activity by the intermediate conductance Ca2+-activated K channel. J Gen Physiol 127(2):159–169. https://doi.org/10.1085/jgp.200509457
Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U (2008) TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455(7217):1210–1215. https://doi.org/10.1038/nature07313
Winston DC, Hennigar RA, Spicer SS, Garrett JR, Schulte BA (1988) Immunohistochemical localization of Na+,K+-ATPase in rodent and human salivary and lacrimal glands. J Histochem Cytochem 36(9):1139–1145. https://doi.org/10.1177/36.9.2841372
Winston DC, Schulte BA, Garrett JR, Proctor GB (1990) Na+, K(+)-ATPase in cat salivary glands and changes induced by nerve stimulation: an immunohistochemical study. J Histochem Cytochem 38(8):1187–1191. https://doi.org/10.1177/38.8.2164061
Won JH, Cottrell WJ, Foster TH, Yule DI (2007) Ca2+ release dynamics in parotid and pancreatic exocrine acinar cells evoked by spatially limited flash photolysis. Am J Physiol Gastrointest Liver Physiol 293:1166–1177
Acknowledgements
The authors thank Timea Horváth and Alexandra Nagy for their excellent technical assistance. This work was supported by the Hungarian National Research Development and Innovation Office (PD112199 to JA and K115307 to PPN) and by the National Institutes of Health grant DE014756. JA is supported by the Janos Bolyai Research Scholarship of the Hungarian Academy of Sciences and the Lajos Szodoray Scholarship of the University of Debrecen. The publication was supported also by the GINOP-2.3.2-15-2016-00040 and EFOP-3.6.2-16-2017-00006 projects (to PPN and JA), which are co-financed by the European Union and the European Regional Development Fund. KM is supported by the Faculty of Dentistry, University of Debrecen.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
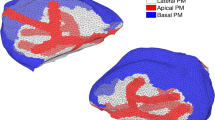
Supplementary figure 1
3D reconstruction of confocal optical sections of an isolated parotid acinus show the localization of the Na+-K+ pump (PDF 609 kb)
Supplementary Material
(DOCX 1478 kb)
Rights and permissions
About this article
Cite this article
Almássy, J., Siguenza, E., Skaliczki, M. et al. New saliva secretion model based on the expression of Na+-K+ pump and K+ channels in the apical membrane of parotid acinar cells. Pflugers Arch - Eur J Physiol 470, 613–621 (2018). https://doi.org/10.1007/s00424-018-2109-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-018-2109-0