Abstract
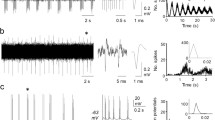
The superficial dorsal horn contains large numbers of interneurons which process afferent and descending information to generate the spinal nociceptive message. Here, we set out to evaluate whether adjustments in patterns and/or temporal correlation of spontaneous discharges of these neurons are involved in the generation of central sensitization caused by peripheral nerve damage. Multielectrode arrays were used to record from discrete groups of such neurons in slices from control or nerve damaged mice. Whole-cell recordings of individual neurons were also obtained. A large proportion of neurons recorded extracellularly showed well-defined patterns of spontaneous firing. Clock-like neurons (CL) showed regular discharges at ∼6 Hz and represented 9 % of the sample in control animals. They showed a tonic-firing pattern to direct current injection and depolarized membrane potentials. Irregular fast-burst neurons (IFB) produced short-lasting high-frequency bursts (2–5 spikes at ∼100 Hz) at irregular intervals and represented 25 % of the sample. They showed bursting behavior upon direct current injection. Of the pairs of neurons recorded, 10 % showed correlated firing. Correlated pairs always included an IFB neuron. After nerve damage, the mean spontaneous firing frequency was unchanged, but the proportion of CL increased significantly (18 %) and many of these neurons appeared to acquire a novel low-threshold A-fiber input. Similarly, the percentage of IFB neurons was unaltered, but synchronous firing was increased to 22 % of the pairs studied. These changes may contribute to transform spinal processing of nociceptive inputs following peripheral nerve damage. The specific roles that these neurons may play are discussed.





Similar content being viewed by others
References
Adrian ED, Zotterman Y (1926) The impulses produced by sensory nerve endings: part 3. Impulses set up by touch and pressure. J Physiol 61:465–483
Adrian ED, Zotterman Y (1926) The impulses produced by sensory nerve-endings: part II. The response of a single end-organ. J Physiol 61:151–171
Averbeck BB, Lee D (2004) Coding and transmission of information by neural ensembles. Trends Neurosci 27:225–230. doi:10.1016/j.tins.2004.02.006
Bailey AL, Ribeiro-da-Silva A (2006) Transient loss of terminals from non-peptidergic nociceptive fibers in the substantia gelatinosa of spinal cord following chronic constriction injury of the sciatic nerve. Neuroscience 138:675–690
Balasubramanyan S, Stemkowski PL, Stebbing MJ, Smith PA (2006) Sciatic chronic constriction injury produces cell-type-specific changes in the electrophysiological properties of rat substantia gelatinosa neurons. J Neurophysiol 96:579–590
Bar-Gad I, Ritov Y, Vaadia E, Bergman H (2001) Failure in identification of overlapping spikes from multiple neuron activity causes artificial correlations. J Neurosci Methods 107:1–13
Bingmer M, Schiemann J, Roeper J, Schneider G (2011) Measuring burstiness and regularity in oscillatory spike trains. J Neurosci Methods 201:426–437. doi:10.1016/j.jneumeth.2011.08.013
Bracci E, Ballerini L, Nistri A (1996) Localization of rhythmogenic networks responsible for spontaneous bursts induced by strychnine and bicuculline in the rat isolated spinal cord. J Neurosci 16:7063–7076
Bromberg-Martin ES, Hikosaka O, Nakamura K (2010) Coding of task reward value in the dorsal raphe nucleus. J Neurosci 30:6262–6272. doi:10.1523/JNEUROSCI.0015-10.2010
Buzsaki G (2004) Large-scale recording of neuronal ensembles. Nat Neurosci 7:446–451. doi:10.1038/nn1233
Cervero F, Iggo A (1980) The substantia gelatinosa of the spinal cord: a critical review. Brain 103:717–772
Cervero F, Iggo A, Ogawa H (1976) Nociceptor-driven dorsal horn neurones in the lumbar spinal cord of the cat. Pain 2:5–24
Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53:55–63
Chapman V, Suzuki R, Dickenson AH (1998) Electrophysiological characterization of spinal neuronal response properties in anaesthetized rats after ligation of spinal nerves L5-L6. J Physiol 507(Pt 3):881–894
Chen Y, Balasubramanyan S, Lai AY, Todd KG, Smith PA (2009) Effects of sciatic nerve axotomy on excitatory synaptic transmission in rat substantia gelatinosa. J Neurophysiol 102:3203–3215. doi:10.1152/jn.00296.2009
Cirillo G, Colangelo AM, Berbenni M, Ippolito VM, De Luca C, Verdesca F, Savarese L, Alberghina L, Maggio N, Papa M (2015) Purinergic modulation of spinal neuroglial maladaptive plasticity following peripheral nerve injury. Mol Neurobiol 52:1440–1457. doi:10.1007/s12035-014-8943-y
Contreras-Hernandez E, Chavez D, Rudomin P (2015) Dynamic synchronization of ongoing neuronal activity across spinal segments regulates sensory information flow. J Physiol 593:2343–2363. doi:10.1113/jphysiol.2014.288134
Corder G, Siegel A, Intondi AB, Zhang X, Zadina JE, Taylor BK (2010) A novel method to quantify histochemical changes throughout the mediolateral axis of the substantia gelatinosa after spared nerve injury: characterization with TRPV1 and substance P. J Pain 11:388–398. doi:10.1016/j.jpain.2009.09.008
Duan B, Cheng L, Bourane S, Britz O, Padilla C, Garcia-Campmany L, Krashes M, Knowlton W, Velasquez T, Ren X, Ross SE, Lowell BB, Wang Y, Goulding M, Ma Q (2014) Identification of spinal circuits transmitting and gating mechanical pain. Cell 159:1417–1432. doi:10.1016/j.cell.2014.11.003
Ecker AS, Berens P, Keliris GA, Bethge M, Logothetis NK, Tolias AS (2010) Decorrelated neuronal firing in cortical microcircuits. Science 327:584–587. doi:10.1126/science.1179867
Fellous JM, Tiesinga PH, Thomas PJ, Sejnowski TJ (2004) Discovering spike patterns in neuronal responses. J Neurosci 24:2989–3001. doi:10.1523/JNEUROSCI.4649-03.2004
Fernandes EC, Luz LL, Mytakhir O, Lukoyanov NV, Szucs P, Safronov BV (2016) Diverse firing properties and Abeta-, Adelta-, and C-afferent inputs of small local circuit neurons in spinal lamina I. Pain 157:475–487. doi:10.1097/j.pain.0000000000000394
Graham BA, Brichta AM, Callister RJ (2004) In vivo responses of mouse superficial dorsal horn neurones to both current injection and peripheral cutaneous stimulation. J Physiol 561:749–763. doi:10.1113/jphysiol.2004.072645
Hains BC, Saab CY, Klein JP, Craner MJ, Waxman SG (2004) Altered sodium channel expression in second-order spinal sensory neurons contributes to pain after peripheral nerve injury. J Neurosci 24:4832–4839. doi:10.1523/JNEUROSCI.0300-04.2004
Izhikevich EM, Desai NS, Walcott EC, Hoppensteadt FC (2003) Bursts as a unit of neural information: selective communication via resonance. Trends Neurosci 26:161–167. doi:10.1016/S0166-2236(03)00034-1
Kohn A, Coen-Cagli R, Kanitscheider I, Pouget A (2016) Correlations and neuronal population information. Annu Rev Neurosci. doi:10.1146/annurev-neuro-070815-013851
Laird JM, Bennett GJ (1993) An electrophysiological study of dorsal horn neurons in the spinal cord of rats with an experimental peripheral neuropathy. J Neurophysiol 69:2072–2085
Li J, Baccei ML (2011) Pacemaker neurons within newborn spinal pain circuits. J Neurosci 31:9010–9022. doi:10.1523/JNEUROSCI.6555-10.2011
Li J, Kritzer E, Ford NC, Arbabi S, Baccei ML (2015) Connectivity of pacemaker neurons in the neonatal rat superficial dorsal horn. J Comp Neurol 523:1038–1053. doi:10.1002/cne.23706
Lisman JE (1997) Bursts as a unit of neural information: making unreliable synapses reliable. Trends Neurosci 20:38–43
Liu XG, Sandkuhler J (1995) The effects of extrasynaptic substance P on nociceptive neurons in laminae I and II in rat lumbar spinal dorsal horn. Neuroscience 68:1207–1218
Lopez-Garcia JA, King AE (1994) Membrane properties of physiologically classified rat dorsal horn neurons in vitro: correlation with cutaneous sensory afferent input. Eur J Neurosci 6:998–1007
Lu Y, Dong H, Gao Y, Gong Y, Ren Y, Gu N, Zhou S, Xia N, Sun YY, Ji RR, Xiong L (2013) A feed-forward spinal cord glycinergic neural circuit gates mechanical allodynia. J Clin Invest 123:4050–4062. doi:10.1172/JCI70026
Luz LL, Szucs P, Safronov BV (2014) Peripherally driven low-threshold inhibitory inputs to lamina I local-circuit and projection neurones: a new circuit for gating pain responses. J Physiol 592:1519–1534. doi:10.1113/jphysiol.2013.269472
Maimon G, Assad JA (2009) Beyond Poisson: increased spike-time regularity across primate parietal cortex. Neuron 62:426–440. doi:10.1016/j.neuron.2009.03.021
Mochizuki Y, Onaga T, Shimazaki H, Shimokawa T, Tsubo Y, Kimura R, Saiki A, Sakai Y, Isomura Y, Fujisawa S, Shibata K, Hirai D, Furuta T, Kaneko T, Takahashi S, Nakazono T, Ishino S, Sakurai Y, Kitsukawa T, Lee JW, Lee H, Jung MW, Babul C, Maldonado PE, Takahashi K, Arce-McShane FI, Ross CF, Sessle BJ, Hatsopoulos NG, Brochier T, Riehle A, Chorley P, Grun S, Nishijo H, Ichihara-Takeda S, Funahashi S, Shima K, Mushiake H, Yamane Y, Tamura H, Fujita I, Inaba N, Kawano K, Kurkin S, Fukushima K, Kurata K, Taira M, Tsutsui K, Ogawa T, Komatsu H, Koida K, Toyama K, Richmond BJ, Shinomoto S (2016) Similarity in neuronal firing regimes across mammalian species. J Neurosci 36:5736–5747. doi:10.1523/JNEUROSCI.0230-16.2016
Nowak LG, Bullier J (2000) Cross correlograms for neuronal spike trains. Different types of temporal correlation in neocortex, their origin and significance. In: Miller R (ed) Time and the brain, conceptual advances in brain research. Harwood Academic, Amsterdam, pp. 53–96
Palecek J, Paleckova V, Dougherty PM, Carlton SM, Willis WD (1992) Responses of spinothalamic tract cells to mechanical and thermal stimulation of skin in rats with experimental peripheral neuropathy. J Neurophysiol 67:1562–1573
Peirs C, Williams SP, Zhao X, Walsh CE, Gedeon JY, Cagle NE, Goldring AC, Hioki H, Liu Z, Marell PS, Seal RP (2015) Dorsal horn circuits for persistent mechanical pain. Neuron 87:797–812. doi:10.1016/j.neuron.2015.07.029
Polgar E, Hughes DI, Arham AZ, Todd AJ (2005) Loss of neurons from laminas I-III of the spinal dorsal horn is not required for development of tactile allodynia in the spared nerve injury model of neuropathic pain. J Neurosci 25:6658–6666
Quiroga RQ, Nadasdy Z, Ben-Shaul Y (2004) Unsupervised spike detection and sorting with wavelets and superparamagnetic clustering. Neural Comput 16:1661–1687. doi:10.1162/089976604774201631
Reali C, Fossat P, Landry M, Russo RE, Nagy F (2011) Intrinsic membrane properties of spinal dorsal horn neurones modulate nociceptive information processing in vivo. J Physiol 589:2733–2743. doi:10.1113/jphysiol.2011.207712
Renart A, de la Rocha J, Bartho P, Hollender L, Parga N, Reyes A, Harris KD (2010) The asynchronous state in cortical circuits. Science 327:587–590. doi:10.1126/science.1179850
Rivera-Arconada I, Lopez-Garcia JA (2015) Characterisation of rebound depolarisation in mice deep dorsal horn neurons in vitro. Pflugers Arch 467:1985–1996. doi:10.1007/s00424-014-1623-y
Rivera-Arconada I, Lopez-Garcia JA (2010) Changes in membrane excitability and potassium currents in sensitized dorsal horn neurons of mice pups. J Neurosci 30:5376–5383. doi:10.1523/JNEUROSCI.4359-09.2010
Rivera-Arconada I, Benedet T, Roza C, Torres B, Barrio J, Krzyzanowska A, Avendano C, Mellstrom B, Lopez-Garcia JA, Naranjo JR (2010) DREAM regulates BDNF-dependent spinal sensitization. Mol Pain 6. doi:10.1186/1744-8069-6-95
Rojas-Piloni G, Dickenson AH, Condes-Lara M (2007) Superficial dorsal horn neurons with double spike activity in the rat. Neurosci Lett 419:147–152
Ruscheweyh R, Ikeda H, Heinke B, Sandkuhler J (2004) Distinctive membrane and discharge properties of rat spinal lamina I projection neurones in vitro. J Physiol 555:527–543. doi:10.1113/jphysiol.2003.054049
Salinas E, Sejnowski TJ (2001) Correlated neuronal activity and the flow of neural information. Nat Rev Neurosci 2:539–550. doi:10.1038/35086012
Sandkuhler J (2009) Models and mechanisms of hyperalgesia and allodynia. Physiol Rev 89:707–758. doi:10.1152/physrev.00025.2008
Sandkuhler J, Eblen-Zajjur AA (1994) Identification and characterization of rhythmic nociceptive and non-nociceptive spinal dorsal horn neurons in the rat. Neuroscience 61:991–1006
Schiemann J, Schlaudraff F, Klose V, Bingmer M, Seino S, Magill PJ, Zaghloul KA, Schneider G, Liss B, Roeper J (2012) K-ATP channels in dopamine substantia nigra neurons control bursting and novelty-induced exploration. Nat Neurosci 15:1272–1280. doi:10.1038/nn.3185
Schoffnegger D, Heinke B, Sommer C, Sandkuhler J (2006) Physiological properties of spinal lamina II GABAergic neurons in mice following peripheral nerve injury. J Physiol 577:869–878. doi:10.1113/jphysiol.2006.118034
Seagrove LC, Suzuki R, Dickenson AH (2004) Electrophysiological characterisations of rat lamina I dorsal horn neurones and the involvement of excitatory amino acid receptors. Pain 108:76–87. doi:10.1016/j.pain.2003.12.004
Shields SD, Eckert WA 3rd, Basbaum AI (2003) Spared nerve injury model of neuropathic pain in the mouse: a behavioral and anatomic analysis. J Pain 4:465–470
Spike RC, Puskar Z, Andrew D, Todd AJ (2003) A quantitative and morphological study of projection neurons in lamina I of the rat lumbar spinal cord. Eur J Neurosci 18:2433–2448
Takaishi K, Eisele JH Jr, Carstens E (1996) Behavioral and electrophysiological assessment of hyperalgesia and changes in dorsal horn responses following partial sciatic nerve ligation in rats. Pain 66:297–306
Tiesinga P, Fellous JM, Sejnowski TJ (2008) Regulation of spike timing in visual cortical circuits. Nat Rev Neurosci 9:97–107. doi:10.1038/nrn2315
Todd AJ (2010) Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci 11:823–836. doi:10.1038/nrn2947
Truini A, Garcia-Larrea L, Cruccu G (2013) Reappraising neuropathic pain in humans—how symptoms help disclose mechanisms. Nat Rev Neurol 9:572–582. doi:10.1038/nrneurol.2013.180
Turrigiano GG (1999) Homeostatic plasticity in neuronal networks: the more things change, the more they stay the same. Trends Neurosci 22:221–227
Willis WD Jr (1999) Dorsal root potentials and dorsal root reflexes: a double-edged sword. Exp Brain Res 124:395–421
XF W, Liu WT, Liu YP, Huang ZJ, Zhang YK, Song XJ (2011) Reopening of ATP-sensitive potassium channels reduces neuropathic pain and regulates astroglial gap junctions in the rat spinal cord. Pain 152:2605–2615. doi:10.1016/j.pain.2011.08.003
Acknowledgments
This study was supported by the Spanish Government (Ministerio de Economía y Competitividad, MINECO; SAF 2016-77585-R) and the Universidad de Alcalá (CCG2015/BIO023).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 367 kb)
Rights and permissions
About this article
Cite this article
Roza, C., Mazo, I., Rivera-Arconada, I. et al. Analysis of spontaneous activity of superficial dorsal horn neurons in vitro: neuropathy-induced changes. Pflugers Arch - Eur J Physiol 468, 2017–2030 (2016). https://doi.org/10.1007/s00424-016-1886-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-016-1886-6