Abstract
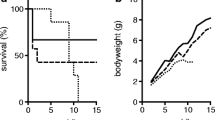
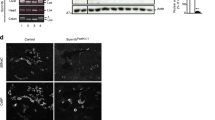
Aldosterone is the main mineralocorticoid hormone controlling sodium balance, fluid homeostasis, and blood pressure by regulating sodium reabsorption in the aldosterone-sensitive distal nephron (ASDN). Germline loss-of-function mutations of the mineralocorticoid receptor (MR) in humans and in mice lead to the “renal” form of type 1 pseudohypoaldosteronism (PHA-1), a case of aldosterone resistance characterized by salt wasting, dehydration, failure to thrive, hyperkalemia, and metabolic acidosis. To investigate the importance of MR in adult epithelial cells, we generated nephron-specific MR knockout mice (MRPax8/LC1) using a doxycycline-inducible system. Under standard diet, MRPax8/LC1 mice exhibit inability to gain weight and significant weight loss compared to control mice. Interestingly, despite failure to thrive, MRPax8/LC1 mice survive but develop a severe PHA-1 phenotype with higher urinary Na+ levels, decreased plasma Na+, hyperkalemia, and higher levels of plasma aldosterone. This phenotype further worsens and becomes lethal under a sodium-deficient diet. Na+/Cl− co-transporter (NCC) protein expression and its phosphorylated form are downregulated in the MRPax8/LC1 knockouts, as well as the αENaC protein expression level, whereas the expression of glucocorticoid receptor (GR) is increased. A diet rich in Na+ and low in K+ does not restore plasma aldosterone to control levels but is sufficient to restore body weight, plasma, and urinary electrolytes. In conclusion, MR deletion along the nephron fully recapitulates the features of severe human PHA‐1. ENaC protein expression is dependent on MR activity. Suppression of NCC under hyperkalemia predominates in a hypovolemic state.









Similar content being viewed by others
Abbreviations
- ASDN:
-
Aldosterone-sensitive distal nephron
- MR:
-
Mineralocorticoid receptor
- GR:
-
Glucocorticoid receptor
- ENaC:
-
Epithelial sodium channel
- NCC:
-
Na+/Cl− co-transporter
- AQP2:
-
Aquaporin 2
- Hsd11b2:
-
11β-Hydroxysteroid dehydrogenase type 2
- PHA-1:
-
Type 1 pseudohypoaldosteronism
- RAAS:
-
Renin-angiotensin-aldosterone system
- PCT:
-
Proximal convoluted tubule
- PST:
-
Proximal straight tubule
- TAL:
-
Thick ascending limb
- DCT:
-
Distal convoluted tubule
- CNT:
-
Connecting tubule
- CCD:
-
Cortical collecting duct
References
Ackermann D, Gresko N, Carrel M, Loffing-Cueni D, Habermehl D, Gomez-Sanchez C, Rossier BC, Loffing J (2010) In vivo nuclear translocation of mineralocorticoid and glucocorticoid receptors in rat kidney: differential effect of corticosteroids along the distal tubule. Am J Physiol Renal Physiol 299:F1473–F1485. doi:10.1152/ajprenal.00437.2010
Arroyo JP, Ronzaud C, Lagnaz D, Staub O, Gamba G (2011) Aldosterone paradox: differential regulation of ion transport in distal nephron. Physiology 26:115–123. doi:10.1152/physiol.00049.2010
Bailey MA, Griffin KJ, Scott DJ (2014) Clinical assessment of patients with peripheral arterial disease. Semin Interv Radiol 31:292–299. doi:10.1055/s-0034-1393964
Balazs Z, Schweizer RA, Frey FJ, Rohner-Jeanrenaud F, Odermatt A (2008) DHEA induces 11β-HSD2 by acting on CCAAT/enhancer-binding proteins. J Am Soc Nephrol 19:92–101. doi:10.1681/ASN.2007030263
Berger S, Bleich M, Schmid W, Cole TJ, Peters J, Watanabe H, Kriz W, Warth R, Greger R, Schutz G (1998) Mineralocorticoid receptor knockout mice: pathophysiology of Na+ metabolism. Proc Natl Acad Sci U S A 95:9424–9429
Berger S, Wolfer DP, Selbach O, Alter H, Erdmann G, Reichardt HM, Chepkova AN, Welzl H, Haas HL, Lipp HP, Schutz G (2006) Loss of the limbic mineralocorticoid receptor impairs behavioral plasticity. Proc Natl Acad Sci U S A 103:195–200. doi:10.1073/pnas.0503878102
Bleich M, Warth R, Schmidt-Hieber M, Schulz-Baldes A, Hasselblatt P, Fisch D, Berger S, Kunzelmann K, Kriz W, Schutz G, Greger R (1999) Rescue of the mineralocorticoid receptor knock-out mouse. Pflugers Arch - Eur J Physiol 438:245–254
Christensen BM, Perrier R, Wang Q, Zuber AM, Maillard M, Mordasini D, Malsure S, Ronzaud C, Stehle JC, Rossier BC, Hummler E (2010) Sodium and potassium balance depends on alphaENaC expression in connecting tubule. J Am Soc Nephrol 21:1942–1951. doi:10.1681/ASN.2009101077
Debonneville C, Flores SY, Kamynina E, Plant PJ, Tauxe C, Thomas MA, Munster C, Chraibi A, Pratt JH, Horisberger JD, Pearce D, Loffing J, Staub O (2001) Phosphorylation of Nedd4-2 by Sgk1 regulates epithelial Na+ channel cell surface expression. EMBO J 20:7052–7059. doi:10.1093/emboj/20.24.7052
Eladari D, Chambrey R, Picard N, Hadchouel J (2014) Electroneutral absorption of NaCl by the aldosterone-sensitive distal nephron: implication for normal electrolytes homeostasis and blood pressure regulation. Cell Mol Life Sci 71:2879–2895. doi:10.1007/s00018-014-1585-4
Funder JW, Feldman D, Edelman IS (1973) The roles of plasma binding and receptor specificity in the mineralocorticoid action of aldosterone. Endocrinology 92:994–1004. doi:10.1210/endo-92-4-994
Gaeggeler HP, Gonzalez-Rodriguez E, Jaeger NF, Loffing-Cueni D, Norregaard R, Loffing J, Horisberger JD, Rossier BC (2005) Mineralocorticoid versus glucocorticoid receptor occupancy mediating aldosterone-stimulated sodium transport in a novel renal cell line. J Am Soc Nephrol 16:878–891. doi:10.1681/ASN.2004121110
Gamba G (2012) Regulation of the renal Na+- Cl− cotransporter by phosphorylation and ubiquitylation. Am J Physiol Renal Physiol 303:F1573–F1583. doi:10.1152/ajprenal.00508.2012
Gomez-Sanchez CE, de Rodriguez AF, Romero DG, Estess J, Warden MP, Gomez-Sanchez MT, Gomez-Sanchez EP (2006) Development of a panel of monoclonal antibodies against the mineralocorticoid receptor. Endocrinology 147:1343–1348. doi:10.1210/en.2005-0860
Gomez-Sanchez EP, Gomez-Sanchez CE (2012) Central regulation of blood pressure by the mineralocorticoid receptor. Mol Cell Endocrinol 350:289–298. doi:10.1016/j.mce.2011.05.005
Koenig JB, Jaffe IZ (2014) Direct role for smooth muscle cell mineralocorticoid receptors in vascular remodeling: novel mechanisms and clinical implications. Curr Hypertens Rep 16:427. doi:10.1007/s11906-014-0427-y
Krozowski ZS, Funder JW (1983) Renal mineralocorticoid receptors and hippocampal corticosterone-binding species have identical intrinsic steroid specificity. Proc Natl Acad Sci U S A 80:6056–6060
Loffing J, Loffing-Cueni D, Valderrabano V, Klausli L, Hebert SC, Rossier BC, Hoenderop JG, Bindels RJ, Kaissling B (2001) Distribution of transcellular calcium and sodium transport pathways along mouse distal nephron. Am J Physiol Renal Physiol 281:F1021–F1027
McGraw AP, McCurley A, Preston IR, Jaffe IZ (2013) Mineralocorticoid receptors in vascular disease: connecting molecular pathways to clinical implications. Curr Atheroscler Rep 15:340. doi:10.1007/s11883-013-0340-x
Messaoudi S, Azibani F, Delcayre C, Jaisser F (2012) Aldosterone, mineralocorticoid receptor, and heart failure. Mol Cell Endocrinol 350:266–272. doi:10.1016/j.mce.2011.06.038
Pressley L, Funder JW (1975) Glucocorticoid and mineralocorticoid receptors in gut mucosa. Endocrinology 97:588–596. doi:10.1210/endo-97-3-588
Rebuffat AG, Tam S, Nawrocki AR, Baker ME, Frey BM, Frey FJ, Odermatt A (2004) The 11-ketosteroid 11-ketodexamethasone is a glucocorticoid receptor agonist. Mol Cell Endocrinol 214:27–37. doi:10.1016/j.mce.2003.11.027
Ronzaud C, Loffing J, Bleich M, Gretz N, Grone HJ, Schutz G, Berger S (2007) Impairment of sodium balance in mice deficient in renal principal cell mineralocorticoid receptor. J Am Soc Nephrol 18:1679–1687. doi:10.1681/ASN.2006090975
Ronzaud C, Loffing J, Gretz N, Schutz G, Berger S (2011) Inducible renal principal cell-specific mineralocorticoid receptor gene inactivation in mice. Am J Physiol Renal Physiol 300:F756–F760. doi:10.1152/ajprenal.00728.2009
Rossier BC, Staub O, Hummler E (2013) Genetic dissection of sodium and potassium transport along the aldosterone-sensitive distal nephron: importance in the control of blood pressure and hypertension. FEBS Lett 587:1929–1941. doi:10.1016/j.febslet.2013.05.013
Rubera I, Loffing J, Palmer LG, Frindt G, Fowler-Jaeger N, Sauter D, Carroll T, McMahon A, Hummler E, Rossier BC (2003) Collecting duct-specific gene inactivation of alphaENaC in the mouse kidney does not impair sodium and potassium balance. J Clin Invest 112:554–565
Rupprecht R, Reul JM, van Steensel B, Spengler D, Soder M, Berning B, Holsboer F, Damm K (1993) Pharmacological and functional characterization of human mineralocorticoid and glucocorticoid receptor ligands. Eur J Pharmacol 247:145–154
Schonig K, Schwenk F, Rajewsky K, Bujard H (2002) Stringent doxycycline dependent control of CRE recombinase in vivo. Nucleic Acids Res 30, e134
Schulz-Baldes A, Berger S, Grahammer F, Warth R, Goldschmidt I, Peters J, Schutz G, Greger R, Bleich M (2001) Induction of the epithelial Na+ channel via glucocorticoids in mineralocorticoid receptor knockout mice. Pflugers Arch - Eur J Physiol 443:297–305. doi:10.1007/s004240100694
Seibert J, Hysek CM, Penno CA, Schmid Y, Kratschmar DV, Liechti ME, Odermatt A (2014) Acute effects of 3,4-methylenedioxymethamphetamine and methylphenidate on circulating steroid levels in healthy subjects. Neuroendocrinology 100:17–25. doi:10.1159/000364879
Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, Ziegler U, Odermatt A, Loffing-Cueni D, Loffing J (2013) Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int 83:811–824. doi:10.1038/ki.2013.14
Traykova-Brauch M, Schonig K, Greiner O, Miloud T, Jauch A, Bode M, Felsher DW, Glick AB, Kwiatkowski DJ, Bujard H, Horst J, von Knebel DM, Niggli FK, Kriz W, Grone HJ, Koesters R (2008) An efficient and versatile system for acute and chronic modulation of renal tubular function in transgenic mice. Nat Med 14:979–984. doi:10.1038/nm.1865
Zhang W, Xia X, Reisenauer MR, Rieg T, Lang F, Kuhl D, Vallon V, Kone BC (2007) Aldosterone-induced Sgk1 relieves Dot1a-Af9-mediated transcriptional repression of epithelial Na+ channel alpha. J Clin Invest 117:773–783. doi:10.1172/JCI29850
Acknowledgments
The authors would like to thank Günter Schütz and Stefan Berger for kindly providing us with the Nr3c2lox/lox mouse line and Celso E. Gomez-Sanchez for the antibody against MR. We also like to thank Denise Kratschmar for LC-MS steroid measurements and Anne-Marie Mérillat for figure editing. Moreover, the authors would like to thank all of the members of the laboratory of Edith Hummler for helpful and interactive discussions. Finally, the authors would like to acknowledge the contribution of the COST Action ADMIRE BM1301.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Animal maintenance and all experimental procedures in mice were in accordance with the Swiss federal guidelines and were approved by the veterinarian local authorities (“Service de la consommation et des affaires vétérinaires”) of the Canton de Vaud, Switzerland.
Conflict of interest
The authors declare that they have no competing interests.
Funding
This work was supported by the Swiss National Center of Competence in Research (NCCR Kidney CH) and the Swiss National Science Foundation (grants SNF 31003A_144198/1 and 31003A_163347/1 to Edith Hummler, 31003A_159454 to Alex Odermatt and 310030_143929 to Johannes Loffing).
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 522 kb)
Rights and permissions
About this article
Cite this article
Canonica, J., Sergi, C., Maillard, M. et al. Adult nephron-specific MR-deficient mice develop a severe renal PHA-1 phenotype. Pflugers Arch - Eur J Physiol 468, 895–908 (2016). https://doi.org/10.1007/s00424-015-1785-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-015-1785-2