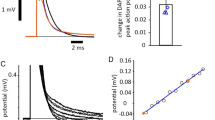
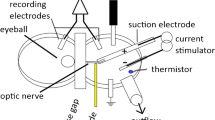
Abstract
Classical work in squid axon reports resting membrane potential is independent of temperature, but our findings suggest that this is not the case for axons in mammalian optic nerve. Refractory period duration changes over 10 times between 37 °C and room temperature, and afterpotential polarity is also acutely temperature sensitive, inconsistent with changes in temperature impacting nerve function only through altered rates of ion channel gating kinetics. Our evidence suggests that the membrane potential is enhanced by warming, an effect reduced by exposure to ouabain. The temperature dependence can be explained if axonal Na+/K+ ATPase continuously expels Na+ ions that enter axons largely electroneutrally, thereby adding a substantial electrogenic component to the membrane potential. Block of the Na+ transporter NKCC1 with bumetanide increases refractoriness, like depolarization, indicating that this is a probable route by which Na+ enters, raising the expectation that the rate of electroneutral Na+ influx increases with temperature and suggesting a temperature-dependent transmembrane Na+ cycle that contributes to membrane potential.








Similar content being viewed by others
Abbreviations
- QTRAC:
-
A computerized threshold-tracking programme available from Digitimer Ltd
- ATX-II:
-
47 Amino acid peptidyl toxin from sea anemone, Anemonia sulcata
- HEPES:
-
2-[4-(2-Hydroxyethyl)piperazin-1-yl]ethanesulfonic acid
- NKCC1:
-
Bumetanide-sensitive Na-K-2Cl co-transporter
- NHE, NHE1:
-
Na-H ion exchanger
- DAP:
-
Depolarizing afterpotential
References
Baker M, Bostock H, Grafe P, Martius P (1987) Function and distribution of three types of rectifying channel in rat spinal root myelinated axons. J Physiol 383(1):45–67
Barrett EF, Barrett JN (1982) Intracellular recording from vertebrate myelinated axons: mechanism of depolarizing afterpotential. J Physiol 323(1):117–144
Belluzzi O, Sacchi O (1986) A quantitative description of the sodium current in the rat sympathetic neurone. J Physiol 380(1):275–291
Blaesse P, Airaksinen MS, Rivera C, Kaila K (2009) Cation-chloride cotransporters and neuronal function. Neuron 61(6):820–838. doi:10.1016/j.neuron.2009.03.003
Boiko T, Rasband MN, Levinson SR, Caldwell JH, Mandel G, Trimmer JS, Matthews G (2001) Compact myelin dictates the differential targeting of two sodium channel isoforms in the same axon. Neuron 30(1):91–104
Boiko T, Van Wart A, Caldwell JH, Levinson SR, Trimmer JS, Matthews G (2003) Functional specialization of the axon initial segment by isoform-specific sodium channel targeting. J Neurosci 23(6):2306–2313
Borst JG, Sakmann B (1998) Calcium current during a single action potential in a large presynaptic terminal of the rat brainstem. J Physiol 506(1):143–157
Bostock H, Baker M, Grafe P, Reid G (1991) Changes in excitability and accommodation of human motor axons following brief periods of ischaemia. J Physiol 441(1):513–535
Caldwell JH, Schaller KL, Lasher RS, Peles E, Levinson SR (2000) Sodium channel NaV1.6 is localized at nodes of Ranvier, dendrites, and synapses. Proc Natl Acad Sci U S A 97(10):5616–5620
Carpenter DO, Alving BO (1968) A contribution of an electrogenic Na+ pump to membrane potential in Aplysia neurons. J Gen Physiol 52(1):1–21
Catterall WA, Cestèle S, Yarov-Yarovoy V, Yu FH, Konoki K, Scheuer T (2007) Voltage-gated ion channels and gating modifier toxins. Toxicon 49(2):124–141. doi:10.1016/j.toxicon.2006.09.022
Ch’en FF-T, Dilworth E, Swietach P, Goddard RS, Vaughan-Jones RD (2003) Temperature dependence of Na+-H+ exchange, Na+-HCO3 co-transport, intracellular buffering and intracellular pH in guinea-pig ventricular myocytes. J Physiol 552(3):715–726
Davis F, Schauf C (1981) Approaches to the development of pharmacological interventions in multiple sclerosis. Adv Neurol 31(1):505–510
Friedrich T, Bamberg E, Nagel G (1996) Na+, K+-ATPase pump currents in giant excised patches activated by an ATP concentration jump. Biophys J 71(5):2486–2500. doi:10.1016/S0006-3495(96)79442-0
Geck P, Pfeiffer B (1985) Na++K++2Cl− cotransport in animal cells—its role in volume regulation. Ann N Y Acad Sci 456(1):166–182
Gordon T, Kocsis J, Waxman S (1990) Electrogenic pump (Na+/K+-ATPase) activity in rat optic nerve. Neuroscience 37(3):829–837
Hakozaki S, Matsumoto M, Sasaki K (1989) Temperature-sensitive activation of G-protein regulating the resting membrane conductance of Aplysia neurons. Jpn J Physiol 39(1):115–130
Halliwell J, Adams P (1982) Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res 250(1):71–92
Hamada K, Matsuura H, Sanada M, Toyoda F, Omatsu-Kanbe M, Kashiwagi A, Yasuda H (2003) Properties of the Na+/K+ pump current in small neurons from adult rat dorsal root ganglia. Br J Pharmacol 138(8):1517–1527. doi:10.1038/sj.bjp.0705170
Herzog RI, Cummins TR, Ghassemi F, Dib-Hajj SD, Waxman SG (2003) Distinct repriming and closed-state inactivation kinetics of NaV1.6 and NaV1.7 sodium channels in mouse spinal sensory neurons. J Physiol 551(3):741–750. doi:10.1113/jphysiol.2003.047357
Hodgkin AL, Huxley AF, Katz B (1952) Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J Physiol 116:424–448
Hodgkin AL, Katz B (1949) The effect of temperature on the electrical activity of the giant axon of the squid. J Physiol 109(1–2):240–249
Hodgkin AL, Katz B (1949) The effect of sodium ions on the electrical activity of the giant axon of the squid. J Physiol 108(1):37–77
James ND, Bartus K, Grist J, Bennett DL, McMahon SB, Bradbury EJ (2011) Conduction failure following spinal cord injury: functional and anatomical changes from acute to chronic stages. J Neurosci 31(50):18543–18555. doi:10.1523/JNEUROSCI. 4306-11.2011
Jang IS, Brodwick MS, Wang ZM, Jeong HJ, Choi BJ, Akaike N (2006) The Na+/H+ exchanger is a major pH regulator in GABAergic presynaptic nerve terminals synapsing onto rat CA3 pyramidal neurons. J Neurochem 99(4):1224–1236. doi:10.1111/j.1471-4159.2006.04168.x
Kang D, Kim D (2006) TREK-2 (K2P10.1) and TRESK (K2P18.1) are major background K+ channels in dorsal root ganglion neurons. Am J Physiol Cell Physiol 291(1):C138–C146. doi:10.1152/ajpcell.00629.2005
Kaplan MR, Cho MH, Ullian EM, Isom LL, Levinson SR, Barres BA (2001) Differential control of clustering of the sodium channels NaV1.2 and NaV1.6 at developing CNS nodes of Ranvier. Neuron 30(1):105–119
Khirug S, Yamada J, Afzalov R, Voipio J, Khiroug L, Kaila K (2008) GABAergic depolarization of the axon initial segment in cortical principal neurons is caused by the Na-K-2Cl cotransporter NKCC1. J Neurosci 28(18):4635–4639. doi:10.1523/JNEUROSCI. 0908-08.2008
Kimelberg HK, Papahadjopoulos D (1974) Effects of phospholipid acyl chain fluidity, and cholesterol on (Na++ K+) -stimulated adenosine triphosphatase. J Biol Chem 249(4):1071–1080
Lai HC, Jan JY (2006) The distribution and targeting of neuronal voltage-gated ion channels. Nat Rev Neurosci 7(7):548–562
Löscher W, Puskarjov M, Kaila K (2013) Cation-chloride cotransporters NKCC1 and KCC2 as potential targets for novel antiepileptic and antiepileptogenic treatments. Neuropharmacology 69(1):62–74. doi:10.1016/j.neuropharm.2012.05.045
Luo J, Chen H, Kintner DB, Shull GE, Sun D (2005) Decreased neuronal death in Na+/H+ exchanger isoform 1 null mice after in vitro and in vivo ischemia. J Neurosci 25(49):11256–11268
Lytle C, McManus T (2002) Coordinate modulation of Na-K-2Cl cotransport and K-Cl cotransport by cell volume and chloride. Am J Physiol Cell Physiol 283(5):C1422–C1431. doi:10.1152/ajpcell.00130.2002
Ma E, Haddad GG (1997) Expression and localization of Na+/H+ exchangers in rat central nervous system. Neuroscience 79(2):591–603
Malek SA, Adorante JS, Stys PK (2005) Differential effects of Na-K-ATPase pump inhibition, chemical anoxia, and glycolytic blockade on membrane potential of rat optic nerve. Brain Res 1037(1–2):171–179. doi:10.1016/j.brainres.2005.01.003
Payne JA, Stevenson TJ, Donaldson LF (1996) Molecular characterization of a putative K-Cl cotransporter in Rat Brain. A neuronal-specific isoform. J Biol Chem 271(27):16245–16252
Rang HP, Ritchie JM (1968) On the electrogenic sodium pump in mammalian non-myelinated nerve fibres and its activation by various external cations. J Physiol 196(1):183–221
Ruffin VA, Salameh AI, Boron WF, Parker MD (2014) Intracellular pH regulation by acid-base transporters in mammalian neurons. Front Physiol 5:43. doi:10.3389/fphys.2014.00043
Salum T, Kõks S, Kairane C, Mahlapuu R, Zilmer M, Vasar E (2010) Temperature dependence of the sodium pump is altered in the cerebral cortex of CCK2 receptor-deficient mice. Neurochem Res 35(5):688–692. doi:10.1007/s11064-009-0119-1
Shen K, Schwartzkroin P (1988) Effects of temperature alterations on population and cellular activities in hippocampal slices from mature and immature rabbit. Brain Res 475(2):305–316
Smith KJ, McDonald WI (1999) The pathophysiology of multiple sclerosis: the mechanisms underlying the production of symptoms and the natural history of the disease. Philos Trans R Soc Lond B Biol Sci 354(1390):1649–1673. doi:10.1098/rstb.1999.0510
Smith KJ, Waxman SG (2005) The conduction properties of demyelinated and remyelinated axons. In: Waxman SG (ed) Mult. Scler. as a Neuronal Dis. Academic, pp 85–100
Snape A, Pittaway JF, Baker MD (2010) Excitability parameters and sensitivity to anemone toxin ATX-II in rat small diameter primary sensory neurones discriminated by Griffonia simplicifolia isolectin IB4. J Physiol 588(1):125–137. doi:10.1113/jphysiol.2009.181107
Thompson SM, Masukawa LM, Prince DA (1985) Temperature dependence of intrinsic membrane properties and synaptic potentials in hippocampal CA1 neurons in vitro. J Neurosci 5(3):817–824
Thuault SJ, Malleret G, Constantinople CM, Nicholls R, Chen I, Zhu J, Panteleyev A, Vronskaya S, Nolan MF, Bruno R, Siegelbaum SA, Kandel ER (2013) Prefrontal cortex HCN1 channels enable intrinsic persistent neural firing and executive memory function. J Neurosci 33(34):13583–13599. doi:10.1523/JNEUROSCI. 2427-12.2013
Todd N, Conn A (1986) Near drowning and hypothermia mimicking severe closed head injury. Br Med J 293(6547):594–595
Tomlinson SE, Tan SV, Kullmann DM, Griggs RC, Burke D, Hanna MG, Bostock H (2010) Nerve excitability studies characterize Kv1.1 fast potassium channel dysfunction in patients with episodic ataxia type 1. Brain 133(12):3530–3540. doi:10.1093/brain/awq318
Urayama O, Shutt H, Sweadner KJ (1989) Identification of three isozyme proteins of the catalytic subunit of the Na, K-ATPase in rat brain. J Biol Chem 264(14):8271–8280
Volgushev M, Vidyasagar TR, Chistiakova M, Yousef T, Eysel UT (2000) Membrane properties and spike generation in rat visual cortical cells during reversible cooling. J Physiol 522(1):59–76
Zeuthen T, MacAulay N (2012) Cotransport of water by Na+-K+-2Cl− cotransporters expressed in Xenopus oocytes: NKCC1 versus NKCC2. J Physiol 590(5):1139–1154. doi:10.1113/jphysiol.2011.226316
Acknowledgments
We acknowledge the support of the MS society (MDB), Barts and the London MSc programme in Translational Neuroscience (GA), and a Rod Flower Scholarship (TAC, JM). We thank Christopher Pendleton for the technical assistance and Peter Reeh, Hugh Bostock, and Monica Calado-Marta for the comments on previous versions of this manuscript.
Compliance with ethical standards statement
Procedures for isolation of ex vivo animal tissue were in accordance with Home Office UK regulations.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Coates, T.A., Woolnough, O., Masters, J.M. et al. Acute temperature sensitivity in optic nerve axons explained by an electrogenic membrane potential. Pflugers Arch - Eur J Physiol 467, 2337–2349 (2015). https://doi.org/10.1007/s00424-015-1696-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-015-1696-2