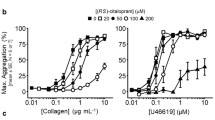
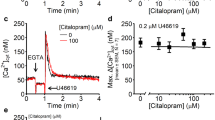
Abstract
Anoctamin 6 (ANO6) is a member of the recently identified TMEM16/anoctamin protein family comprising Ca2+-activated Cl− channels that generate outward-rectifying ionic currents in response to intracellular Ca2+ increase. ANO6 is also essential for Ca2+-dependent phospholipid scrambling required for blood coagulation. Selective serotonin reuptake inhibitors (SSRIs)—fluoxetine, sertraline, and paroxetine—that are used for the treatment of major depressive disorders can increase the risk of upper gastrointestinal bleeding after chronic treatment. However, at the earlier stage of intake, which is 1–7 days after the treatment, the possibility of blood coagulation might also increase, but transiently. Therefore, in this study, we investigated whether therapeutic SSRI concentrations affected the Cl− current or phospholipid scrambling activity of ANO6 by assessing ANO6 currents (I ANO6), phosphatidylserine (PS) exposure, and platelet aggregation. In the whole-cell patch mode, SSRIs facilitated Ca2+-dependent activation of IANO6 in ANO6-transfected cells, as evidenced by a significant decrease in the delay of IANO6 generation. On the other hand, in the inside-out patch clamp configuration, SSRIs showed an inhibitory effect on ANO6 currents, suggesting that SSRIs activate ANO6 via an indirect mechanism in intact cells. SSRIs also facilitated Ca2+-dependent PS exposure and α-thrombin-induced platelet aggregation. These results indicate that SSRIs at clinically relevant concentrations promote Ca2+-dependent activation of ANO6, which may have potential clinical implications such as the underlying mechanism of SSRI-induced adverse drug reactions.








Similar content being viewed by others
References
Altamura AC, Moro AR, Percudani M (1994) Clinical pharmacokinetics of fluoxetine. Clin Pharmacokinet 26(3):201–214. doi:10.2165/00003088-199426030-00004
Baumann P (1996) Pharmacokinetic-pharmacodynamic relationship of the selective serotonin reuptake inhibitors. Clin Pharmacokinet 31(6):444–469. doi:10.2165/00003088-199631060-00004
Baumann P (1996) Pharmacology and pharmacokinetics of citalopram and other SSRIs. Int Clin Psychopharmacol 11(Suppl 1):5–11
Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ (2008) TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322(5901):590–594. doi:10.1126/science.1163518
Choi JS, Hahn SJ, Rhie DJ, Yoon SH, Jo YH, Kim MS (1999) Mechanism of fluoxetine block of cloned voltage-activated potassium channel Kv1.3. J Pharmacol Exp Ther 291(1):1–6
de Abajo FJ, Montero D, Rodriguez LA, Madurga M (2006) Antidepressants and risk of upper gastrointestinal bleeding. Basic Clin Pharmacol Toxicol 98(3):304–310. doi:10.1111/j.1742-7843.2006.pto_303.x
Deak F, Lasztoczi B, Pacher P, Petheo GL, Valeria K, Spat A (2000) Inhibition of voltage-gated calcium channels by fluoxetine in rat hippocampal pyramidal cells. Neuropharmacology 39(6):1029–1036
Dilks JR, Flaumenhaft R (2008) Fluoxetine (Prozac) augments platelet activation mediated through protease-activated receptors. J Thromb Haemost 6(4):705–708. doi:10.1111/j.1538-7836.2008.02896.x
Duvvuri U, Shiwarski DJ, Xiao D, Bertrand C, Huang X, Edinger RS, Rock JR, Harfe BD, Henson BJ, Kunzelmann K, Schreiber R, Seethala RS, Egloff AM, Chen X, Lui VW, Grandis JR, Gollin SM (2012) TMEM16A induces MAPK and contributes directly to tumorigenesis and cancer progression. Cancer Res 72(13):3270–3281. doi:10.1158/0008-5472.CAN-12-0475-T
Edgar VA, Genaro AM, Cremaschi G, Sterin-Borda L (1998) Fluoxetine action on murine T-lymphocyte proliferation: participation of PKC activation and calcium mobilisation. Cell Signal 10(10):721–726
Edgar VA, Sterin-Borda L, Cremaschi GA, Genaro AM (1999) Role of protein kinase C and cAMP in fluoxetine effects on human T-cell proliferation. Eur J Pharmacol 372(1):65–73
Fumagalli F, Molteni R, Calabrese F, Frasca A, Racagni G, Riva MA (2005) Chronic fluoxetine administration inhibits extracellular signal-regulated kinase 1/2 phosphorylation in rat brain. J Neurochem 93(6):1551–1560. doi:10.1111/j.1471-4159.2005.03149.x
Grubb S, Poulsen KA, Juul CA, Kyed T, Klausen TK, Larsen EH, Hoffmann EK (2013) TMEM16F (Anoctamin 6), an anion channel of delayed Ca(2+) activation. J Gen Physiol 141(5):585–600. doi:10.1085/jgp.201210861
Halperin D, Reber G (2007) Influence of antidepressants on hemostasis. Dialogues Clin Neurosci 9(1):47–59
Harper CM, Fukodome T, Engel AG (2003) Treatment of slow-channel congenital myasthenic syndrome with fluoxetine. Neurology 60(10):1710–1713
Harper MT, Poole AW (2013) Chloride channels are necessary for full platelet phosphatidylserine exposure and procoagulant activity. Cell Death Dis 19(4):e969. doi:10.1038/cddis.2013.495
Heurteaux C, Lucas G, Guy N, El Yacoubi M, Thummler S, Peng XD, Noble F, Blondeau N, Widmann C, Borsotto M, Gobbi G, Vaugeois JM, Debonnel G, Lazdunski M (2006) Deletion of the background potassium channel TREK-1 results in a depression-resistant phenotype. Nat Neurosci 9(9):1134–1141. doi:10.1038/nn1749
Hiemke C, Hartter S (2000) Pharmacokinetics of selective serotonin reuptake inhibitors. Pharmacol Ther 85(1):11–28
Huang F, Wang X, Ostertag EM, Nuwal T, Huang B, Jan YN, Basbaum AI, Jan LY (2013) TMEM16C facilitates Na(+)-activated K(+) currents in rat sensory neurons and regulates pain processing. Nat Neurosci 16(9):1284–1290. doi:10.1038/nn.3468
Hwang SJ, Blair PJ, Britton FC, O’Driscoll KE, Hennig G, Bayguinov YR, Rock JR, Harfe BD, Sanders KM, Ward SM (2009) Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J Physiol 587(Pt 20):4887–4904. doi:10.1113/jphysiol.2009.176198
Jonnakuty C, Gragnoli C (2008) What do we know about serotonin? J Cell Physiol 217(2):301–306. doi:10.1002/jcp.21533
Jung J, Nam JH, Park HW, Oh U, Yoon JH, Lee MG (2013) Dynamic modulation of ANO1/TMEM16A HCO3(−) permeability by Ca2+/calmodulin. Proc Natl Acad Sci U S A 110(1):360–365. doi:10.1073/pnas.1211594110
Kasper S, Dotsch M, Kick H, Vieira A, Moller HJ (1993) Plasma concentrations of fluvoxamine and maprotiline in major depression: implications on therapeutic efficacy and side effects. Eur Neuropsychopharmacol: J Eur Neuropsychopharmacol 3(1):13–21. doi:10.1016/0924-977X(93)90290-3
Kennard LE, Chumbley JR, Ranatunga KM, Armstrong SJ, Veale EL, Mathie A (2005) Inhibition of the human two-pore domain potassium channel, TREK-1, by fluoxetine and its metabolite norfluoxetine. Br J Pharmacol 144(6):821–829. doi:10.1038/sj.bjp.0706068
Kobayashi T, Washiyama K, Ikeda K (2003) Inhibition of G protein-activated inwardly rectifying K+ channels by fluoxetine (Prozac). Br J Pharmacol 138(6):1119–1128. doi:10.1038/sj.bjp.0705172
Maertens C, Droogmans G, Verbesselt R, Nilius B (2002) Block of volume-regulated anion channels by selective serotonin reuptake inhibitors. Naunyn Schmiedeberg’s Arch Pharmacol 366(2):158–165. doi:10.1007/s00210-002-0567-5
Maertens C, Wei L, Voets T, Droogmans G, Nilius B (1999) Block by fluoxetine of volume-regulated anion channels. Br J Pharmacol 126(2):508–514. doi:10.1038/sj.bjp.0702314
Martins JR, Faria D, Kongsuphol P, Reisch B, Schreiber R, Kunzelmann K (2011) Anoctamin 6 is an essential component of the outwardly rectifying chloride channel. Proc Natl Acad Sci U S A 108(44):18168–18172. doi:10.1073/pnas.1108094108
Mercier G, Lennon AM, Renouf B, Dessouroux A, Ramauge M, Courtin F, Pierre M (2004) MAP kinase activation by fluoxetine and its relation to gene expression in cultured rat astrocytes. J Mol Neurosci 24(2):207–216. doi:10.1385/JMN:24:2:207
Ousingsawat J, Kongsuphol P, Schreiber R, Kunzelmann K (2011) CFTR and TMEM16A are separate but functionally related Cl− channels. Cell Physiol Biochem 28(4):715–724. doi:10.1159/000335765
Pancrazio JJ, Kamatchi GL, Roscoe AK, Lynch C 3rd (1998) Inhibition of neuronal Na+ channels by antidepressant drugs. J Pharmacol Exp Ther 284(1):208–214
Park HW, Nam JH, Kim JY, Namkung W, Yoon JS, Lee JS, Kim KS, Venglovecz V, Gray MA, Kim KH, Lee MG (2010) Dynamic regulation of CFTR bicarbonate permeability by [Cl−]i and its role in pancreatic bicarbonate secretion. Gastroenterology 139(2):620–631. doi:10.1053/j.gastro.2010.04.004
Pifferi S, Dibattista M, Menini A (2009) TMEM16B induces chloride currents activated by calcium in mammalian cells. Pflugers Arch 458(6):1023–1038. doi:10.1007/s00424-009-0684-9
Rock JR, O’Neal WK, Gabriel SE, Randell SH, Harfe BD, Boucher RC, Grubb BR (2009) Transmembrane protein 16A (TMEM16A) is a Ca2+-regulated Cl− secretory channel in mouse airways. J Biol Chem 284(22):14875–14880. doi:10.1074/jbc.C109.000869
Romanenko VG, Catalan MA, Brown DA, Putzier I, Hartzell HC, Marmorstein AD, Gonzalez-Begne M, Rock JR, Harfe BD, Melvin JE (2010) Tmem16A encodes the Ca2+-activated Cl− channel in mouse submandibular salivary gland acinar cells. J Biol Chem 285(17):12990–13001. doi:10.1074/jbc.M109.068544
Ruggeri ZM (2002) Platelets in atherothrombosis. Nat Med 8(11):1227–1234. doi:10.1038/nm1102-1227
Sauer WH, Berlin JA, Kimmel SE (2001) Selective serotonin reuptake inhibitors and myocardial infarction. Circulation 104(16):1894–1898
Schroeder BC, Cheng T, Jan YN, Jan LY (2008) Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 134(6):1019–1029
Shimizu T, Iehara T, Sato K, Fujii T, Sakai H, Okada Y (2013) TMEM16F is a component of a Ca2+-activated Cl− channel but not a volume-sensitive outwardly rectifying Cl− channel. Am J Physiol Cell Physiol 304(8):C748–C759. doi:10.1152/ajpcell.00228.2012
Stephan AB, Shum EY, Hirsh S, Cygnar KD, Reisert J, Zhao H (2009) ANO2 is the cilial calcium-activated chloride channel that may mediate olfactory amplification. Proc Natl Acad Sci U S A 106(28):11776–11781. doi:10.1073/pnas.0903304106
Suzuki J, Nagata S (2011) Phospholipid scrambling by TMEM16F. Seikagaku 83(11):1050–1054
Suzuki J, Umeda M, Sims PJ, Nagata S (2010) Calcium-dependent phospholipid scrambling by TMEM16F. Nature 468(7325):834–838. doi:10.1038/nature09583
Tata LJ, West J, Smith C, Farrington P, Card T, Smeeth L, Hubbard R (2005) General population based study of the impact of tricyclic and selective serotonin reuptake inhibitor antidepressants on the risk of acute myocardial infarction. Heart 91(4):465–471. doi:10.1136/hrt.2004.037457
Tian Y, Schreiber R, Kunxelmann K (2012) Anoctamins are a family of Ca2+ activated Cl- channels. J Cell Sci 125(pt 21):4991–4998. doi:10.1242/jcs.10955
Traboulsie A, Chemin J, Kupfer E, Nargeot J, Lory P (2006) T-type calcium channels are inhibited by fluoxetine and its metabolite norfluoxetine. Mol Pharmacol 69(6):1963–1968. doi:10.1124/mol.105.020842
Wernicke JF (2004) Safety and side effect profile of fluoxetine. Expert Opin Drug Saf 3(5):495–504. doi:10.1517/14740338.3.5.495
Wong DT, Bymaster FP, Engleman EA (1995) Prozac (fluoxetine, Lilly 110140), the first selective serotonin uptake inhibitor and an antidepressant drug: twenty years since its first publication. Life Sci 57(5):411–441
Wong DT, Perry KW, Bymaster FP (2005) Case history: the discovery of fluoxetine hydrochloride (Prozac). Nat Rev Drug Discov 4(9):764–774. doi:10.1038/nrd1821
Yang H, Kim A, David T, Palmer D, Jin T, Tien J, Huang F, Cheng T, Coughlin SR, Jan YN, Jan LY (2012) TMEM16F forms a Ca2+-activated cation channel required for lipid scrambling in platelets during blood coagulation. Cell 151(1):111–122. doi:10.1016/j.cell.2012.07.036
Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U (2008) TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455(7217):1210–1215. doi:10.1038/nature07313
Yeung SY, Millar JA, Mathie A (1999) Inhibition of neuronal KV potassium currents by the antidepressant drug, fluoxetine. Br J Pharmacol 128(7):1609–1615. doi:10.1038/sj.bjp.0702955
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2011-0014404, to J.H.N) and by grants 2013R1A3A2042197 and 2007-0056092 from the National Research Foundation, the Ministry of Science, ICT and Future Planning, Korea (to M.G.L).
Conflict of interest
The authors state no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Hyun Jong Kim and Ikhyun Jun contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Ca2+-dependent activation kinetics of hANO1. a-b Typical IANO1 traces in the whole-cell patch clamp with symmetrical NMDG-Cl (150 mM) and 200 nM free Ca2+ in the pipette solution and the related I-V curves in hANO1-overexpressing HEK293T cells (n = 6). I-V relationship was assessed for each event by voltage ramps from -100 mV to +100 mV at the rate (dV/dt) = 0.2 V/s. Note that IANO1 was generated immediately after the start of the whole-cell recording. (DOC 199 kb)
Supplementary Fig. 2
ANO6 expression and whole-cell patch clamp in mock-transfected HEK293T cells. a Comparison of ANO6 expression between mock- and ANO6-transfected HEK293T cells by immunoblotting analysis. ANO6 expression was detected only after long exposure of the membranes. b Representative traces of mock-transfected HEK293T cells in the whole-cell patch clamp with 10 μM free Ca2+ pipette solution. Note that IANO6 was not generated during the recording time (over 20 min). c Relative I-V relationship. (DOC 766 kb)
Supplementary Fig. 3
Inhibitory effects of SSRIs on ANO1 activation in HEK293T cells. IANO1 was inhibited by SSRIs in both whole-cell and inside-out patch clamps. The whole-cell patch clamp recording from hANO1-expressing HEK293T cells was performed using symmetrical (150 mM) NMDG-Cl (bath and pipette); the pipette solution contained 200 nM Ca2+ for ANO1 activation. For the inside-out patch clamp recording, the patches were exposed to 300 nM Ca2+, with symmetrical (150 mM) NMDG-Cl (left-side panels). I-V relationship was obtained by voltage ramps from −100 to +100 mV at the rate (dV/dt) = 0.2 V/s; the holding potential was 0 mV (right-side panels). Representative traces of fluoxetine (F), sertraline (S), and paroxetine (P) on IANO1 in inside-out (a, d, e) and whole-cell (b, d) patch clamp recording. The data are the results of three independent experiments. (DOC 175 kb)
Supplementary Fig. 4
Fluoxetine potentiates ANO6-like currents in PANC-1 cells. a Representative traces showing the effect of 300 μM fluoxetine on IANO6-like current traces with 3 μM Ca2+ pipette solution. b Mean I-V relationship curve obtained before fluoxetine treatment and during the application of the peak current after fluoxetine treatment. Data are presented as means ± SE (n = 7). (DOC 175 kb)
Supplementary Fig. 5
Fluoxetine at 100 μM induces PS exposure in both mock- and ANO6-transfected HEK293T cells. PS exposure of mock-transfected (a) or ANO6-transfected (b)HEK293T cells pre-treated with 100 μM fluoxetine for 10 min and then treated with of 1 μM ionomycin was detected by flow cytometry using Annexin V-FITC staining. Note that PS exposure occurred in both mock- and ANO6-transfected HEK293T cells (right panels). (DOC 774 kb)
Rights and permissions
About this article
Cite this article
Kim, H.J., Jun, I., Yoon, J.S. et al. Selective serotonin reuptake inhibitors facilitate ANO6 (TMEM16F) current activation and phosphatidylserine exposure. Pflugers Arch - Eur J Physiol 467, 2243–2256 (2015). https://doi.org/10.1007/s00424-015-1692-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-015-1692-6