Abstract
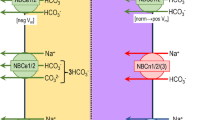
Sulfate is essential for normal physiology. The kidney plays a major role in sulfate homeostasis. Sulfate is freely filtered and strongly reabsorbed in the proximal tubule. The apical membrane Na+–sulfate cotransporter NaS1 (SLC13A1) mediates sulfate (re)absorption across renal proximal tubule and small intestinal epithelia. NaS1 encodes a 595-amino acid (≈66 kDa) protein with 13 putative transmembrane domains. Its substrate preferences are sodium and sulfate, thiosulfate, and selenate, and its activity is inhibited by molybdate, selenate, tungstate, thiosulfate, succinate, and citrate. NaS1 is primarily expressed in the kidney (proximal tubule) and intestine (duodenum to colon). NaS1 expression is down-regulated in the renal cortex by high sulfate diet, hypothyroidism, vitamin D depletion, glucocorticoids, hypokalemia, metabolic acidosis, and NSAIDs and up-regulated by low sulfate diet, thyroid hormone, vitamin D supplementation, growth hormone, chronic renal failure, and during post-natal growth. Disruption of murine NaS1 gene leads to hyposulfatemia and hypersulfaturia, as well as changes in metabolism, growth, fecundity, behavior, gut physiology, and liver detoxification. This suggests that NaS1 is an important sulfate transporter and its disruption leads to perturbed sulfate homeostasis, which contributes to numerous pathophysiological conditions.



Similar content being viewed by others
References
Aronson PS (1989) The renal proximal tubule: a model for diversity of anion exchangers and stilbene-sensitive anion transporters. Annu Rev Plant Physiol Plant Mol Biol 51:419–441
Bastlein C, Burckhardt G (1986) Sensitivity of rat renal luminal and contraluminal sulfate transport to DIDS. Am J Physiol 250:F226–F234
Beck L, Markovich D (2000) The mouse Na(+)–sulfate cotransporter gene Nas1. Cloning, tissue distribution, gene structure, chromosomal assignment, and transcriptional regulation by vitamin D. J Biol Chem 275:11880–11890
Bissig M, Hagenbuch B, Stieger B, Koller T, Meier PJ (1994) Functional expression cloning of the canalicular sulfate transport system of rat hepatocytes. J Biol Chem 269:3017–3021
Brazy PC, Dennis VW (1981) Sulfate transport in rabbit proximal convoluted tubules: presence of anion exchange. Am J Physiol 241:F300–F307
Busch AE, Waldegger S, Herzer T, Biber J, Markovich D, Murer H, Lang F (1994) Electrogenic cotransport of Na+ and sulfate in Xenopus oocytes expressing the cloned Na+SO4(2-) transport protein NaSi-1. J Biol Chem 269:12407–12409
Cole DE, Baldwin LS, Stirk LJ (1984) Increased inorganic sulfate in mother and fetus at parturition: evidence for a fetal-to-maternal gradient. Am J Obstet Gynecol 148:596–599
David C, Ullrich KJ (1992) Substrate specificity of the luminal Na+-dependent sulphate transport system in the proximal renal tubule as compared to the contraluminal sulphate exchange system. Pflugers Arch 421:455–465
Dawson PA, Beck L, Markovich D (2003) Hyposulfatemia, growth retardation, reduced fertility and seizures in mice lacking a functional NaSi-1 gene. Proc Natl Acad Sci U S A 100:13704–13709
Dawson PA, Choyce A, Chuang C, Whitelock J, Markovich D, Leggatt GR (2010) Enhanced tumor growth in the NaS1 sulfate transporter null mouse. Cancer Sci 101:369–373
Dawson PA, Gardiner B, Grimmond S, Markovich D (2006) Transcriptional profile reveals altered hepatic lipid and cholesterol metabolism in hyposulfatemic NaS1 null mice. Physiol Genomics 26:116–124
Dawson PA, Gardiner B, Lee S, Grimmond S, Markovich D (2008) Kidney transcriptome reveals altered steroid homeostasis in NaS1 sulfate transporter null mice. J Steroid Biochem Mol Biol 112:55–62
Dawson PA, Huxley S, Gardiner B, Tran T, McAuley JL, Grimmond S, McGuckin MA, Markovich D (2009) Reduced mucin sulfonation and impaired intestinal barrier function in the hyposulfataemic NaS1 null mouse. Gut 58:910–919
Dawson PA, Markovich D (2002) Regulation of the mouse Nas1 promoter by vitamin D and thyroid hormone. Pflugers Arch 444:353–359
Dawson PA, Markovich D (2005) Pathogenetics of the human SLC26 transporters. Curr Med Chem 12:385–396
Dawson PA, Russell CS, Lee S, McLeay SC, van Dongen JM, Cowley DM, Clarke LA, Markovich D (2010) Urolithiasis and hepatotoxicity are linked to the anion transporter Sat1 in mice. J Clin Invest 120:706–712
Dawson P, Sim P, Simmons D, Markovich D (2011) Fetal loss and hyposulfataemia in pregnant NaS1 transporter null mice. J Reprod Dev 57:444–449
Dawson PA, Steane SE, Markovich D (2004) Behavioural abnormalities of the hyposulphataemic Nas1 knock-out mouse. Behav Brain Res 154:457–463
Dawson PA, Steane SE, Markovich D (2005) Impaired memory and olfactory performance in NaSi-1 sulphate transporter deficient mice. Behav Brain Res 159:15–20
Fernandes I, Hampson G, Cahours X, Morin P, Coureau C, Couette S, Prie D, Biber J, Murer H, Friedlander G, Silve C (1997) Abnormal sulfate metabolism in vitamin D-deficient rats. J Clin Invest 100:2196–2203
Fernandes I, Laouari D, Tutt P, Hampson G, Friedlander G, Silve C (2001) Sulfate homeostasis, NaSi-1 cotransporter, and SAT-1 exchanger expression in chronic renal failure in rats. Kidney Int 59:210–221
Florin T, Neale G, Gibson GR, Christl SU, Cummings JH (1991) Metabolism of dietary sulphate: absorption and excretion in humans. Gut 32:766–773
Goudsmit A Jr, Power MH, Bollman JL (1939) The excretion of sulfates by the dog. Am J Physiol 125:506
Hagenbuch B, Stange G, Murer H (1985) Transport of sulphate in rat jejunal and proximal tubular basolateral membrane vesicles. Pflugers Arch 405:202–208
Hierholzer K, Cade R, Gurd R, Kessler R, Pitts R (1960) Stop flow analysis of renal reabsorption and excretion of sulfate in the dog. Am J Physiol 198:833–837
Karniski LP, Lotscher M, Fucentese M, Hilfiker H, Biber J, Murer H (1998) Immunolocalization of sat-1 sulfate/oxalate/bicarbonate anion exchanger in the rat kidney. Am J Physiol 275:F79–F87
Kuo SM, Aronson PS (1988) Oxalate transport via the sulfate/HCO3 exchanger in rabbit renal basolateral membrane vesicles. J Biol Chem 263:9710–9717
Lee A, Beck L, Markovich D (2000) The human renal sodium sulfate cotransporter (SLC13A1; hNaSi-1) cDNA and gene: organization, chromosomal localization, and functional characterization. Genomics 70:354–363
Lee S, Dawson PA, Hewavitharana AK, Shaw PN, Markovich D (2006) Disruption of NaS1 sulfate transport function in mice leads to enhanced acetaminophen-induced hepatotoxicity. Hepatology 43:1241–1247
Lee A, Dawson PA, Markovich D (2005) NaSi-1 and Sat-1: Structure, function and transcriptional regulation of two genes encoding renal proximal tubular sulfate transporters. Int J Biochem Cell Biol 37:1350–1356
Lee S, Kesby JP, Muslim MD, Steane SE, Eyles DW, Dawson PA, Markovich D (2007) Hyperserotonaemia and reduced brain serotonin levels in NaS1 sulphate transporter null mice. Neuroreport 18:1981–1985
Lee A, Markovich D (2004) Characterization of the human renal Na(+)-sulphate cotransporter gene ( NAS1) promoter. Pflugers Arch 448:490–499
Lee HJ, Sagawa K, Shi W, Murer H, Morris ME (2000) Hormonal regulation of sodium/sulfate co-transport in renal epithelial cells. Proc Soc Exp Biol Med 225:49–57
Lotscher M, Custer M, Quabius ES, Kaissling B, Murer H, Biber J (1996) Immunolocalization of Na/SO4-cotransport (NaSi-1) in rat kidney. Pflugers Arch - Eur J Physiol 432:373–378
Low I, Friedrich T, Burckhardt G (1984) Properties of an anion exchanger in rat renal basolateral membrane vesicles. Am J Physiol 246:F334–F342
Lücke H, Stange G, Murer H (1979) Sulphate-ion/sodium-ion co-transport by brush-border membrane vesicles isolated from rat kidney cortex. Biochem J 182:223–229
Markovich D (2001) Physiological roles and regulation of mammalian sulfate transporters. Physiol Rev 81:1499–1533
Markovich D (2008) Expression cloning and radiotracer uptakes in Xenopus laevis oocytes. Nat Protoc 3:1975–1980
Markovich D, Aronson PS (2007) Specificity and regulation of renal sulfate transporters. Annu Rev Physiol 69:361–375
Markovich D, Forgo J, Stange G, Biber J, Murer H (1993) Expression cloning of rat renal Na+/SO4(2-) cotransport. Proc Natl Acad Sci U S A 90:8073–8077
Markovich D, Murer H, Biber J, Sakhaee K, Pak C, Levi M (1998) Dietary sulfate regulates the expression of the renal brush border Na/Si cotransporter NaSi-1. J Am Soc Nephrol 9:1568–1573
Markovich D, Wang H, Puttaparthi K, Zajicek H, Rogers T, Murer H, Biber J, Levi M (1999) Chronic K depletion inhibits renal brush border membrane Na/sulfate cotransport. Kidney Int 55:244–251
Markovich D, Werner A, Murer H (1999) Expression cloning with Xenopus oocytes. In: Hildebrandt F, Igarashi P (eds) Techniques in molecular medicine. Springer, Heidelberg, pp 310–318
Morris ME, Levy G (1983) Plasma inorganic sulfate concentrations in pregnant women. J Pharm Sci 72:715–716
Pritchard JB, Renfro JL (1983) Renal sulfate transport at the basolateral membrane is mediated by anion exchange. Proc Natl Acad Sci U S A 80:2603–2607
Puttaparthi K, Markovich D, Halaihel N, Wilson P, Zajicek HK, Wang H, Biber J, Murer H, Rogers T, Levi M (1999) Metabolic acidosis regulates rat renal Na-Si cotransport activity. Am J Physiol 276:C1398–C1404
Regeer RR, Markovich D (2004) A dileucine motif targets the sulfate anion transporter sat-1 to the basolateral membrane in renal cell lines. Am J Physiol Cell Physiol 287:C365–C372
Sagawa K, Benincosa LJ, Murer H, Morris ME (1998) Ibuprofen-induced changes in sulfate renal transport. J Pharmacol Exp Ther 287:1092–1097
Sagawa K, DuBois DC, Almon RR, Murer H, Morris ME (1998) Cellular mechanisms of renal adaptation of sodium dependent sulfate cotransport to altered dietary sulfate in rats. J Pharmacol Exp Ther 287:1056–1062
Sagawa K, Han B, DuBois DC, Murer H, Almon RR, Morris ME (1999) Age- and growth hormone-induced alterations in renal sulfate transport. J Pharmacol Exp Ther 290:1182–1187
Sagawa K, Murer H, Morris ME (1999) Effect of experimentally induced hypothyroidism on sulfate renal transport in rats. Am J Physiol 276:F164–F171
Schneider EG, Durham JC, Sacktor B (1984) Sodium-dependent transport of inorganic sulfate by rabbit renal brush-border membrane vesicles. Effects of other ions. J Biol Chem 259:14591–14599
Turner RJ (1984) Sodium-dependent sulfate transport in renal outer cortical brush border membrane vesicles. Am J Physiol 247:F793–F798
Ullrich KJ, Murer H (1982) Sulphate and phosphate transport in the renal proximal tubule. Philos Trans R Soc B 299:549–558
Ullrich KJ, Rumrich G (1988) Contraluminal transport systems in the proximal renal tubule involved in secretion of organic anions. Am J Physiol 254:F453–F462
Ullrich KJ, Rumrich G, Kloess S (1980) Bidirectional active transport of thiosulfate in the proximal convolution of the rat kidney. Pflugers Arch 387:127–132
Ullrich KJ, Rumrich G, Kloess S (1984) Contraluminal sulfate transport in the proximal tubule of the rat kidney. I. Kinetics, effects of K+, Na+, Ca2+, H+ and anions. Pflugers Arch 402:264–271
Ullrich KJ, Rumrich G, Kloess S (1985) Contraluminal sulfate transport in the proximal tubule of the rat kidney. II. Specificity: sulfate-ester, sulfonates and amino sulfonates. Pflugers Arch 404:293–299
Ullrich KJ, Rumrich G, Kloess S (1985) Contraluminal sulfate transport in the proximal tubule of the rat kidney. III. Specificity: disulfonates, di- and tri-carboxylates and sulfo-carboxylates. Pflugers Arch 404:300–306
Ullrich KJ, Rumrich G, Kloess S (1985) Contraluminal sulfate transport in the proximal tubule of the rat kidney. IV. Specificity: salicylate analogs. Pflugers Arch 404:307–310
Ullrich KJ, Rumrich G, Kloess S (1985) Contraluminal sulfate transport in the proximal tubule of the rat kidney. V. Specificity: phenophthaleins, sulfonphthaleins and other sulfodyes, sulfamoyl compounds and dyphenylamine-2-carboxylates. Pflugers Arch 404:311–318
Acknowledgments
Research in the author's laboratory has been supported by the Australian National Health and Medical Research Council, the Australian Research Council, the Cancer Council Queensland, and the University of Queensland.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published as part of the Special Issue on “Sodium-coupled transporters in health and disease.”
Rights and permissions
About this article
Cite this article
Markovich, D. Na+–sulfate cotransporter SLC13A1. Pflugers Arch - Eur J Physiol 466, 131–137 (2014). https://doi.org/10.1007/s00424-013-1388-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-013-1388-8