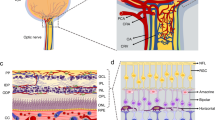
Abstract
Pericytes in the retina differ from pericytes in many other organs by their high density and their cooperative role in the neurovascular unit. Their diverse ontogeny and the fact that not one pericyte marker identifies the entire population suggest also functional plurality in the retina, including invading cells of mesenchymal origin. Further, to establish factors determining pericyte recruitment, modifiers of pericyte adhesion and homeostasis, such as notch-3 and angptl-4, have been recently identified, expanding the understanding of pericyte function in the retina. Also, the role of pericytes as part of the neurovascular unit has been appreciated, given that the neuroglia determines pericyte survival and motility under disease conditions. Pericyte dropout is not unique in the diabetic retina, and non-diabetic animal models may prove useful in the search for mechanisms involved in disease-associated dysfunction of the neurovascular unit.




Similar content being viewed by others
References
Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ (2010) Structure and function of the blood–brain barrier. Neurobiol Dis 37:13–25
Alikhani M, Roy S, Graves DT (2010) Foxo1 plays an essential role in apoptosis of retinal pericytes. Mol Vis 16:408–415
Allende ML, Yamashita T, Proia RL (2003) G-protein-coupled receptor S1p1 acts within endothelial cells to regulate vascular maturation. Blood 102:3665–3667
Armulik A, Genove G, Betsholtz C (2011) Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21:193–215
Armulik A, Abramsson A, Betsholtz C (2005) Endothelial/pericyte interactions. Circ Res 97:512–523
Balabanov R, Washington R, Wagnerova J, Dore-Duffy P (1996) CNS microvascular pericytes express macrophage-like function, cell surface integrin alpha M, and macrophage marker Ed-2. Microvasc Res 52:127–142
Behl Y, Krothapalli P, Desta T, Dipiazza A, Roy S, Graves DT (2008) Diabetes-enhanced tumor necrosis factor-alpha production promotes apoptosis and the loss of retinal microvascular cells in type 1 and type 2 models of diabetic retinopathy. Am J Pathol 172:1411–1418
Bringmann A, Iandiev I, Pannicke T, Wurm A, Hollborn M, Wiedemann P et al (2009) Cellular signaling and factors involved in Muller cell gliosis: neuroprotective and detrimental effects. Prog Retin Eye Res 28:423–451
Cai J, Kehoe O, Smith GM, Hykin P, Boulton ME (2008) The angiopoietin/Tie-2 system regulates pericyte survival and recruitment in diabetic retinopathy. Invest Ophthalmol Vis Sci 49:2163–2171
Cai X, Lin Y, Friedrich CC, Neville C, Pomerantseva I, Sundback CA et al (2009) Bone marrow derived pluripotent cells are pericytes which contribute to vascularization. Stem Cell Rev 5:437–445
Cardoso FL, Brites D, Brito MA (2010) Looking at the blood–brain barrier: molecular anatomy and possible investigation approaches. Brain Res Rev 64:328–363
Cogan DG, Toussaint D, Kuwabara T (1961) Retinal vascular patterns. IV. Diabetic retinopathy. Arch Ophthalmol 66:366–378
Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS et al (2008) A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3:301–313
Da Silva Meirelles L, Caplan AI, Nardi NB (2008) In search of the in vivo identity of mesenchymal stem cells. Stem Cells 26:2287–2299
De Smet F, Segura I, De Bock K, Hohensinner PJ, Carmeliet P (2009) Mechanisms of vessel branching: filopodia on endothelial tip cells lead the way. Arterioscler Thromb Vasc Biol 29:639–649
Dejana E, Tournier-Lasserve E, Weinstein BM (2009) The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev Cell 16:209–221
Diaz-Flores L, Gutierrez R, Madrid JF, Varela H, Valladares F, Acosta E et al (2009) Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol 24:909–969
Enge M, Bjarnegard M, Gerhardt H, Gustafsson E, Kalen M, Asker N et al (2002) Endothelium-specific platelet-derived growth factor-B ablation mimics diabetic retinopathy. EMBO J 21:4307–4316
Farrington-Rock C, Crofts NJ, Doherty MJ, Ashton BA, Griffin-Jones C, Canfield AE (2004) Chondrogenic and adipogenic potential of microvascular pericytes. Circulation 110:2226–2232
Feng Y, Pfister F, Schreiter K, Wang Y, Stock O, Vom Hagen F et al (2008) Angiopoietin-2 deficiency decelerates age-dependent vascular changes in the mouse retina. Cell Physiol Biochem 21:129–136
Feng Y, Vom Hagen F, Wang Y, Beck S, Schreiter K, Pfister F et al (2009) The absence of angiopoietin-2 leads to abnormal vascular maturation and persistent proliferative retinopathy. Thromb Haemost 102:120–130
Fruttiger M (2002) Development of the mouse retinal vasculature: angiogenesis versus vasculogenesis. Invest Ophthalmol Vis Sci 43:522–527
Gaengel K, Genove G, Armulik A, Betsholtz C (2009) Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol 29:630–638
Geraldes P, Hiraoka-Yamamoto J, Matsumoto M, Clermont A, Leitges M, Marette A et al (2009) Activation of Pkc-delta and Shp-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat Med 15:1298–1306
Gerhardt H, Betsholtz C (2003) Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res 314:15–23
Hamilton NB, Attwell D, Hall CN (2010) Pericyte-mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Front Neuroenergetics 2:5
Hammes HP, Feng Y, Pfister F, Brownlee M (2011) Diabetic retinopathy: targeting vasoregression. Diabetes 60:9–16
Hammes HP, Du X, Edelstein D, Taguchi T, Matsumura T, Ju Q et al (2003) Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med 9:294–299
Hammes HP, Federoff HJ, Brownlee M (1995) Nerve growth factor prevents both neuroretinal programmed cell death and capillary pathology in experimental diabetes. Mol Med 1:527–534
Hammes HP, Lin J, Renner O, Shani M, Lundqvist A, Betsholtz C et al (2002) Pericytes and the pathogenesis of diabetic retinopathy. Diabetes 51:3107–3112
Hammes HP, Lin J, Wagner P, Feng Y, Vom Hagen F, Krzizok T et al (2004) Angiopoietin-2 causes pericyte dropout in the normal retina: evidence for involvement in diabetic retinopathy. Diabetes 53:1104–1110
Heglind M, Cederberg A, Aquino J, Lucas G, Ernfors P, Enerback S (2005) Lack of the central nervous system- and neural crest-expressed forkhead gene Foxs1 affects motor function and body weight. Mol Cell Biol 25:5616–5625
Holderfield MT, Hughes CC (2008) Crosstalk between vascular endothelial growth factor, notch, and transforming growth factor-beta in vascular morphogenesis. Circ Res 102:637–652
Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P et al (1996) Notch3 mutations in cadasil, a hereditary adult-onset condition causing stroke and dementia. Nature 383:707–710
Kern TS, Engerman RL (1995) Vascular lesions in diabetes are distributed non-uniformly within the retina. Exp Eye Res 60:545–549
Kern TS, Tang J, Berkowitz BA (2010) Validation of structural and functional lesions of diabetic retinopathy in mice. Mol Vis 16:2121–2131
Kovac A, Erickson MA, Banks WA (2011) Brain microvascular pericytes are immunoactive in culture: cytokine, chemokine, nitric oxide, and Lrp-1 expression in response to lipopolysaccharide. J Neuroinflammation 8:139
Krenning G, Moonen JR, Van Luyn MJ, Harmsen MC (2008) Vascular smooth muscle cells for use in vascular tissue engineering obtained by endothelial-to-mesenchymal transdifferentiation (EnMT) on collagen matrices. Biomaterials 29:3703–3711
Kuwabara T, Cogan DG (1960) Studies of retinal vascular patterns. I. Normal architecture. Arch Ophthalmol 64:904–911
Lewandowska E, Szpak GM, Wierzba-Bobrowicz T, Modzelewska J, Stepien T, Pasennik E et al (2010) Capillary vessel wall in CADASIL angiopathy. Folia Neuropathol 48:104–115
Li F, Lan Y, Wang Y, Wang J, Yang G, Meng F et al (2011) Endothelial Smad4 maintains cerebrovascular integrity by activating N-cadherin through cooperation with Notch. Dev Cell 20:291–302
Li W, Yanoff M, Liu X, Ye X (1997) Retinal capillary pericyte apoptosis in early human diabetic retinopathy. Chin Med J (Engl) 110:659–663
Lindahl P, Johansson BR, Leveen P, Betsholtz C (1997) Pericyte loss and microaneurysm formation in pdgf-B-deficient mice. Science 277:242–245
Liu H, Zhang W, Kennard S, Caldwell RB, Lilly B (2010) Notch3 is critical for proper angiogenesis and mural cell investment. Circ Res 107:860–870
Ma X, Robin C, Ottersbach K, Dzierzak E (2002) The Ly-6a (Sca-1) Gfp transgene is expressed in All adult mouse hematopoietic stem cells. Stem Cells 20:514–521
Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C et al (1997) Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277:55–60
Majesky MW (2007) Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol 27:1248–1258
Mcguire PG, Rangasamy S, Maestas J, Das A (2011) Pericyte-derived sphingosine 1-phosphate induces the expression of adhesion proteins and modulates the retinal endothelial cell barrier. Arterioscler Thromb Vasc Biol 31:e107–115
Mcleod DS, Hasegawa T, Prow T, Merges C, Lutty G (2006) The initial fetal human retinal vasculature develops by vasculogenesis. Dev Dyn 235:3336–3347
Merfeld-Clauss S, Gollahalli N, March KL, Traktuev DO (2010) Adipose tissue progenitor cells directly interact with endothelial cells to induce vascular network formation. Tissue Eng Part A 16:2953–2966
Moonen JR, Krenning G, Brinker MG, Koerts JA, Van Luyn MJ, Harmsen MC (2010) Endothelial progenitor cells give rise to pro-angiogenic smooth muscle-like progeny. Cardiovasc Res 86:506–515
Nehls V, Drenckhahn D (1991) Heterogeneity of microvascular pericytes for smooth muscle type alpha-actin. J Cell Biol 113:147–154
Peppiatt CM, Howarth C, Mobbs P, Attwell D (2006) Bidirectional control of Cns capillary diameter by pericytes. Nature 443:700–704
Perdiguero EG, Galaup A, Durand M, Teillon J, Philippe J, Valenzuela DM et al (2011) Alteration of developmental and pathological retinal angiogenesis in Angptl4-deficient mice. J Biol Chem 286:36841–36851
Pfister F, Feng Y, Vom Hagen F, Hoffmann S, Molema G, Hillebrands JL et al (2008) Pericyte migration: a novel mechanism of pericyte loss in experimental diabetic retinopathy. Diabetes 57:2495–2502
Puro DG (2007) Physiology and pathobiology of the pericyte-containing retinal microvasculature: new developments. Microcirculation 14:1–10
Rajantie I, Ilmonen M, Alminaite A, Ozerdem U, Alitalo K, Salven P (2004) Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood 104:2084–2086
Romeo G, Liu WH, Asnaghi V, Kern TS, Lorenzi M (2002) Activation of nuclear factor-kappab induced by diabetes and high glucose regulates a proapoptotic program in retinal pericytes. Diabetes 51:2241–2248
Ruchoux MM, Guerouaou D, Vandenhaute B, Pruvo JP, Vermersch P, Leys D (1995) Systemic vascular smooth muscle cell impairment in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Acta Neuropathol 89:500–512
Rucker HK, Wynder HJ, Thomas WE (2000) Cellular mechanisms of CNS pericytes. Brain Res Bull 51:363–369
Sa-Pereira I, Brites D, Brito MA (2012) Neurovascular unit: a focus on pericytes. Mol Neurobiol 45:327–347
Schor AM, Allen TD, Canfield AE, Sloan P, Schor SL (1990) Pericytes derived from the retinal microvasculature undergo calcification in vitro. J Cell Sci 97(Pt 3):449–461
Shepro D, Morel NM (1993) Pericyte physiology. FASEB J 7:1031–1038
Simonavicius N, Ashenden M, Van Weverwijk A, Lax S, Huso DL, Buckley CD et al (2012) Pericytes promote selective vessel regression to regulate vascular patterning. Blood 120:1516–1527
Sims DE (2000) Diversity within pericytes. Clin Exp Pharmacol Physiol 27:842–846
Sorrell JM, Baber MA, Traktuev DO, March KL, Caplan AI (2011) The creation of an in vitro adipose tissue that contains a vascular-adipocyte complex. Biomaterials 32:9667–9676
Stalmans I, Ng YS, Rohan R, Fruttiger M, Bouche A, Yuce A et al (2002) Arteriolar and venular patterning in retinas of mice selectively expressing vegf isoforms. J Clin Invest 109:327–336
Sundberg C, Kowanetz M, Brown LF, Detmar M, Dvorak HF (2002) Stable expression of angiopoietin-1 and other markers by cultured pericytes: phenotypic similarities to a subpopulation of cells in maturing vessels during later stages of angiogenesis in vivo. Lab Invest 82:387–401
Tidhar A, Reichenstein M, Cohen D, Faerman A, Copeland NG, Gilbert DJ et al (2001) A novel transgenic marker for migrating limb muscle precursors and for vascular smooth muscle cells. Dev Dyn 220:60–73
Traktuev DO, Merfeld-Clauss S, Li J, Kolonin M, Arap W, Pasqualini R et al (2008) A population of multipotent Cd34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res 102:77–85
Van Deurs B (1976) Observations on the blood–brain barrier in hypertensive rats, with particular reference to phagocytic pericytes. J Ultrastruct Res 56:65–77
Yao D, Taguchi T, Matsumura T, Pestell R, Edelstein D, Giardino I et al (2007) High glucose increases angiopoietin-2 transcription in microvascular endothelial cells through methylglyoxal modification of Msin3a. J Biol Chem 282:31038–31045
Yatoh S, Mizutani M, Yokoo T, Kozawa T, Sone H, Toyoshima H et al (2006) Antioxidants and an inhibitor of advanced glycation ameliorate death of retinal microvascular cells in diabetic retinopathy. Diabetes Metab Res Rev 22:38–45
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pfister, F., Przybyt, E., Harmsen, M.C. et al. Pericytes in the eye. Pflugers Arch - Eur J Physiol 465, 789–796 (2013). https://doi.org/10.1007/s00424-013-1272-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-013-1272-6