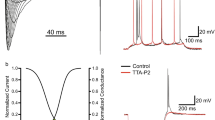
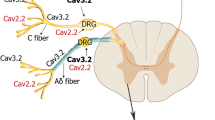
Abstract
Pain is an important clinical problem and, in its chronic form, may be a disabling condition. Most currently available therapies are insufficient and/or accompanied by serious side effects. Recent studies have implicated the CaV3.2 isoform of T-type Ca channels in nociceptive signaling. CaV3.2 channels are located in the somas of dorsal root ganglion cells and in the central endings of these cells in the dorsal horn of the spinal cord. These channels can support the development and maintenance of both physiological (nociceptive) and pathological (neuropathic) pain. In this review, we summarize the most recent evidence linking the presynaptic CaV3.2 channels to the etiology of neuropathic pain disorders. In particular, we focus on data linking plasticity of CaV3.2 channels with neuropathic pain disorders associated with mechanical peripheral nerve injury and with diabetic peripheral neuropathy. We also discuss the development of potential pain therapies aimed at these channels.

Similar content being viewed by others
References
Basbaum AI, Bautista DM, Scherrer G, Julius D (2009) Cellular and molecular mechanisms of pain. Cell 139(2):267–284
Bourinet E, Alloui A, Monteiol A, Barrere C, Couette B, Poirot O, Pages A, McRory J, Snutch TP, Eschalier A, Nargeot J (2005) Silencing of the Cav3.2 T-type calcium channel gene in sensory neurons demonstrates its major role in nociception. EMBO J 24:315–324
Bossu JL, Feltz A, Thomann JM (1985) Depolarization elicits two distinct calcium currents in vertebrate sensory neurons. Pflugers Arch 403:360–368
Cao XH, Byun HS, Chen SR, Pan HL (2011) Diabetic neuropathy enhances voltage-activated Ca2+ channel activity and its control by M4 muscarinic receptors in primary sensory neurons. J Neurochem 119(3):594–603
Carbone E, Lux HD (1984) A low-voltage activated, fully inactivating Ca2+ channel in vertebrate sensory neurons. Nature 310:501–502
Cardenas CG, Del Mar LP, Scroggs RS (1995) Variation in serotonergic inhibition of calcium channel currents in four types of rat sensory neurons differentiated by membrane properties. J Neurophysiol 74:1870–1879
Coderre TJ, Katz J, Vaccarino AL, Melzack R (1993) Contribution of central neuroplasticity to pathological pain: review of clinical and experimental evidence. Pain 52:259–285
Choe WJ, Messinger RB, Leach E, Eckle V-S, Obradovic A, Salajegheh R, Jevtovic-Todorovic V, Todorovic SM (2011) TTA-P2 is a potent and selective blocker of T-type calcium channels in rat sensory neurons and a novel antinociceptive agent. Mol Pharmacol 80(5):900–910
Choi S, Na HS, Kim J, Lee J, Lee S, Kim D, Park J, Chen CC, Campbell KP, Shin HS (2007) Attenuated pain responses in mice lacking CaV3.2 T-type channels. Genes Brain Behav 6(5):425–431
Chen CC, Lamping KG, Nuno DW, Barresi R, Prouty SJ, Lavoie JL, Cribbs LL, England SK, Sigmund CD, Weiss RM, Williamson RA, Hill JA, Campbell CP (2003) Abnormal coronary function in mice deficient in alpha1H T-type Ca2+ channels. Science 302:1416–1418
Coste B, Crest M, Delmas P (2007) Pharmacological dissection and distribution of NaN/Nav1.9, T-type Ca2+ currents, and mechanically activated cation currents in different populations of DRG neurons. J Gen Physiol 129(1):57–77
Dubreuil AS, Boukhaddaoui H, Desmadryl G, Martinez-Salgado C, Moshourab R, Lewin GR, Carroll P, Valmier J, Scamps F (2004) Role of T-type calcium current in identified d-hair mechanoreceptor neurons studied in vitro. J Neurosci 24:8480–8484
Dogrul A, Gardell LR, Ossipov MH, Tulunay FC, Lai J, Porecca F (2003) Reversal of experimental neuropathic pain by T-type calcium channel blockers. Pain 105:159–168
Edwards JL, Vincent AM, Cheng HT, Feldman EL (2008) Diabetic neuropathy: mechanisms to management. Pharmacol Ther 120:1–34
Fedulova SA, Kostuyak PG, Veselovsky NS (1985) Two types of calcium channels in the somatic membrane of new-born rat dorsal root ganglion neurons. J Physiol (London) 359:431–446
Fox AP, Nowycky MC, Tsien RW (1987) Single-channel recordings of three types of calcium channels in chick sensory neurones. J Physiol (London) 394:173–200
Flatters SJ, Bennett GJ (2004) Ethosuximide reverses paclitaxel- and vincristine-induced painful peripheral neuropathy. Pain 109(1–2):150–161
Gooch C, Podwall D (2004) The diabetic neuropathies. Neurologist 10:311–322
Hall KE, Sima AA, Wiley JW (1995) Voltage-dependent calcium currents are enhanced in dorsal root ganglion neurones from the Bio Bred/Worchester diabetic rat. J Physiol (London) 486(Pt 2):313–322
Ikeda H, Stark J, Fischer H, Wagner M, Drdla R, Jäger T, Sandkühler J (2006) Synaptic amplifier of inflammatory pain in the spinal dorsal horn. Science 312(5780):1659–1662
Jacus MO, Uebele VN, Renger JJ, Todorovic SM (2012) Presynaptic CaV3.2 channels regulate excitatory neurotransmission in nociceptive dorsal horn neurons. J Neurosci 32(27):9374–9382
Jagodic MM, Pathirathna S, Nelson MT, Mancuso S, Joksovic PM, Rosenberg ER, Bayliss DA, Jevtovic-Todorovic V, Todorovic SM (2007) Cell-specific alterations of T-type calcium current in painful diabetic neuropathy enhance excitability of sensory neurons. J Neurosci 27(12):3305–3316
Jagodic MM, Pathirathna S, Joksovic PM, Lee W, Nelson MT, Naik AK, Su P, Jevtovic-Todorovic V, Todorovic SM (2008) Up-regulation of the T-type calcium current in small rat sensory neurons after chronic constrictive injury of the sciatic nerve. J Neurophysiol 99:3151–3156
Latham JR, Pathirathna S, Jagodic MM, Choe WJ, Levin ME, Nelson MT, Lee WY, Krishnan K, Covey D, Todorovic SM, Jevtovic-Todorovic V (2009) Selective T-type calcium channel blockade alleviates hyperalgesia in ob/ob mice. Diabetes 58(11):2656–2665
Levine JD, Reichling DB (1999) Peripheral mechanisms of inflammatory pain. In: Wall PD, Melzack R (eds) Textbook of pain, 4th edn. Churchill Livingstone, London, pp 59–84
McCallum JB, Kwok WM, Mynlieff M, Bosnjak ZJ, Hogan QH (2003) Loss of T-type calcium current in sensory neurons of rats with neuropathic pain. Anesthesiol 98:209–216
Messinger RB, Naik AK, Jagodic MM, Nelson MT, Lee WY, Choe WJ, Orestes P, Latham JR, Todorovic SM, Jevtovic-Todorovic V (2009) In-vivo silencing of the Cav3.2 T-type calcium channels in sensory neurons alleviates hyperalgesia in rats with streptozocin-induced diabetic neuropathy. Pain 145(1-2):184–195
Nelson MT, Joksovic PM, Perez-Reyes E, Todorovic SM (2005) The endogenous redox agent L-cysteine induces T-type Ca2+ channel-dependent sensitization of a novel subpopulation of rat peripheral nociceptors. J Neurosci 25:8766–8775
Nelson MT, Woo J, Kang H-W, Barrett PQ, Vitko J, Perez-Reyes E, Lee J-H, Shin H-S, Todorovic SM (2007) Reducing agents sensitize C-type nociceptors by relieving high-affinity zinc inhibition of T-type calcium channels. J Neurosci 27(31):8250–8260
Okubo K, Matsumura M, Kawaishi Y, Aoki Y, Matsunami M, Okawa Y, Sekiguchi F, Kawabata A (2012) Hydrogen sulfide-induced mechanical hyperalgesia and allodynia require activation of both CaV3.2 and TRPA1 channels in mice. Br J Pharmacol 166(5):1738–1743
Pathirathna S, Brimelow BC, Jagodic MM, Kathiresan K, Jiang X, Zorumski CF, Mennerick S, Covey DF, Todorovic SM, Jevtovic-Todorovic V (2005) New evidence that both T-type Ca2+ channels and GABAA channels are responsible for the potent peripheral analgesic effects of 5α-reduced neuroactive steroids. Pain 114:429–443
Pathirathna S, Todorovic SM, Covey DF, Jevtovic-Todorovic V (2005) 5α-reduced neuroactive steroids alleviate thermal and mechanical hyperalgesia in rats with neuropathic pain. Pain 117:326–339
Shin JB, Martinez-Salgado C, Heppenstall PA, Lewin GR (2003) A T-type calcium channel required for normal function of a mammalian mechanoreceptor. Nat Neurosci 6(7):724–730
Takahashi T, Aoki Y, Okubo K, Maeda Y, Sekiguchi F, Mitani K, Nishikawa H, Kawabata A (2010) Upregulation of Ca(v)3.2 T-type calcium channels targeted by endogenous hydrogen sulfide contributes to maintenance of neuropathic pain. Pain 150(1):183–191
Talley EM, Cribbs LL, Lee JH, Daud A, Perez-Reyes E, Bayliss DA (1999) Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels. J Neurosci 19:1895–1911
Todorovic SM, Lingle CJ (1998) Pharmacological properties of T-type Ca2+ current in adult rat sensory neurons: Effects of anticonvulsant and anesthetic agents. J Neurophysiol 79:240–252
Todorovic SM, Jevtovic-Todorovic V, Meyenburg A, Mennerick S, Perez-Reyes E, Romano C, Olney JW, Zorumski CF (2001) Redox modulation of T-type calcium channels in rat peripheral nociceptors. Neuron 31:75–85
Todorovic SM, Prakriya M, Nakashima YM, Nilsson KR, Han M, Zorumski CF, Covey DF, Lingle CJ (1998) Enantioselective blockade of t-type Ca2+ current in adult rat sensory neurons by a steroid that lacks γ-aminobutyric acid-modulatory activity. Mol Pharmacol 54:918–927
Todorovic SM, Meyenburg A, Jevtovic-Todorovic V (2004) Redox modulation of peripheral T-type Ca2+ channels in vivo: alteration of nerve injury-induced thermal hyperalgesia. Pain 109:328–339
Todorovic SM, Pathirathna S, Brimelow BC, Jagodic MM, Ko SH, Jiang X, Nilsson KR, Zorumski CF, Covey DF, Jevtovic-Todorovic V (2004) 5β−reduced neuroactive steroids are novel voltage-dependent blockers of T-type Ca2+ channels in rat sensory neurons in vitro and potent peripheral analgesics in vivo. Mol Pharmacol 66:1223–1235
White G, Lovinger DM, Weight FF (1989) Transient low-threshold Ca2+ current triggers burst firing through an afterdepolarizing potential in an adult mammalian neuron. PNAS USA 86:6802–6806
Yue J, Liu L, Liu Z, Shu B, Zhang Y (2012) Upregulation of T-type Ca2+ channels in primary sensory neurons in spinal nerve injury. Spine 2012 Epub ahead of print
Acknowledgments
Our research is supported by American Diabetes Association National Award for Basic Research 7-09-BS-190 (to S.M.T.), Dr. Harold Carron Endowment fund (to V.J-T.) and research funds from the Department of Anesthesiology at the University of Virginia, Charlottesville, VA
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Todorovic, S.M., Jevtovic-Todorovic, V. Neuropathic pain: role for presynaptic T-type channels in nociceptive signaling. Pflugers Arch - Eur J Physiol 465, 921–927 (2013). https://doi.org/10.1007/s00424-012-1211-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-012-1211-y