Abstract
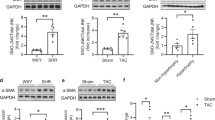
N–Acetyl–seryl–aspartyl–lysyl–proline (Ac-SDKP) inhibits endothelin-1 (ET-1)-induced activation of p44/42 mitogen-activated protein kinase (p44/42 MAPK) and collagen production in cultured rat cardiac fibroblasts (RCFs). However, we do not know whether its inhibitory effect on p44/42 MAPK is due to the altered activity of protein tyrosine phosphatases (PTPs), which in turn downregulate the p44/42 MAPK signaling pathway. The activity of Src homology 2-containing protein tyrosine phosphatase-2 (SHP-2) is downregulated by ET-1 in RCFs; thus, we hypothesized that Ac-SDKP inhibits ET-1-stimulated collagen production in part by preserving SHP-2 activity and thereby inhibiting p44/42 MAPK phosphorylation. When we stimulated RCFs with ET-1 in the presence or absence of Ac-SDKP, we found that (a) PTP activity was reduced by ET-1 and (b) this effect was counteracted by Ac-SDKP in a dose-dependent fashion. Next, we extracted SHP-2 from RCF lysates by immunoprecipitation and determined that (a) ET-1 inhibited SHP-2 by 40 % and (b) this effect was prevented by Ac-SDKP. However, Ac-SDKP failed to inhibit ET-1-induced p44/42 MAPK phosphorylation in RCFs treated with SHP-2 short hairpin RNA (shRNA); in contrast, in cells transfected with control shRNA, Ac-SDKP’s inhibitory effect on ET-1-induced p44/42 MAPK activation remained intact. Moreover, the inhibitory effect of Ac-SDKP on ET-1-stimulated collagen production was blunted in cells treated with the SHP-1/2 inhibitor NSC-87877. Thus, we concluded that the inhibitory effect of Ac-SDKP on ET-1-stimulated collagen production by RCFs is mediated in part by preserving SHP-2 activity and thereby preventing p44/42 MAPK activation. Ac-SDKP or its analogs could represent a new therapeutic tool to treat fibrotic diseases in the cardiovascular system.







Similar content being viewed by others
References
Ammarguellat F, Larouche I, Schiffrin EL (2001) Myocardial fibrosis in DOCA-salt hypertensive rats: effect of endothelin ET(A) receptor antagonism. Circulation 103:319–324
Azizi M, Rousseau A, Ezan E, Guyene TT, Michelet S, Grognet JM, Lenfant M, Corvol P, Menard J (1996) Acute angiotensin-converting enzyme inhibition increases the plasma level of the natural stem cell regulator N–acetyl–seryl–aspartyl–lysyl–proline. J Clin Invest 97:839–844
Brondello JM, Brunet A, Pouyssegur J, McKenzie FR (1997) The dual specificity mitogen-activated protein kinase phosphatase-1 and -2 are induced by the p42/p44MAPK cascade. J Biol Chem 272:1368–1376
Chen CH, Cheng TH, Lin H, Shih NL, Chen YL, Chen YS, Cheng CF, Lian WS, Meng TC, Chiu WT, Chen JJ (2006) Reactive oxygen species generation is involved in epidermal growth factor receptor transactivation through the transient oxidization of Src homology 2-containing tyrosine phosphatase in endothelin-1 signaling pathway in rat cardiac fibroblasts. Mol Pharmacol 69:1347–1355
Chen L, Sung SS, Yip ML, Lawrence HR, Ren Y, Guida WC, Sebti SM, Lawrence NJ, Wu J (2006) Discovery of a novel shp2 protein tyrosine phosphatase inhibitor. Mol Pharmacol 70:562–570
Chiariello M, Ambrosio G, Cappelli-Bigazzi M, Perrone-Filardi P, Brigante F, Sifola C (1986) A biochemical method for the quantitation of myocardial scarring after experimental coronary artery occlusion. J Mol Cell Cardiol 18:283–290
Cleutjens JP, Verluyten MJ, Smiths JF, Daemen MJ (1995) Collagen remodeling after myocardial infarction in the rat heart. Am J Pathol 147:325–338
Cramer H, Schmenger K, Heinrich K, Horstmeyer A, Boning H, Breit A, Piiper A, Lundstrom K, Muller-Esterl W, Schroeder C (2001) Coupling of endothelin receptors to the ERK/MAP kinase pathway. Roles of palmitoylation and G(alpha)q. Eur J Biochem 268:5449–5459
Eghbali M, Tomek R, Sukhatme VP, Woods C, Bhambi B (1991) Differential effects of transforming growth factor-beta 1 and phorbol myristate acetate on cardiac fibroblasts. Regulation of fibrillar collagen mRNAs and expression of early transcription factors. Circ Res 69:483–490
Groom LA, Sneddon AA, Alessi DR, Dowd S, Keyse SM (1996) Differential regulation of the MAP, SAP and RK/p38 kinases by Pyst1, a novel cytosolic dual-specificity phosphatase. EMBO J 15:3621–3632
Guarda E, Katwa LC, Myers PR, Tyagi SC, Weber KT (1993) Effects of endothelins on collagen turnover in cardiac fibroblasts. Cardiovasc Res 27:2130–2134
Hafizi S, Wharton J, Chester AH, Yacoub MH (2004) Profibrotic effects of endothelin-1 via the ETA receptor in cultured human cardiac fibroblasts. Cell Physiol Biochem 14:285–292
Haneda M, Sugimoto T, Kikkawa R (1999) Mitogen-activated protein kinase phosphatase: a negative regulator of the mitogen-activated protein kinase cascade. Eur J Pharmacol 365:1–7
Huang WQ, Wang QR (2001) Bone marrow endothelial cells secrete thymosin β4 and AcSDKP. Exp Hematol 29:12–18
Matozaki T, Murata Y, Saito Y, Okazawa H, Ohnishi H (2009) Protein tyrosine phosphatase SHP-2: a proto-oncogene product that promotes Ras activation. Cancer Sci 100:1786–1793
Meszaros JG, Gonzalez AM, Endo-Mochizuki Y, Villegas S, Villarreal F, Brunton LL (2000) Identification of G protein-coupled signaling pathways in cardiac fibroblasts: cross talk between G(q) and G(s). AM J Physiol Cell Physiol 278:C154–C162
Park JB, Schiffrin EL (2002) Cardiac and vascular fibrosis and hypertrophy in aldosterone-infused rats: role of endothelin-1. Am J Hypertens 15:164–169
Peng H, Carretero OA, Liao TD, Peterson EL, Rhaleb N-E (2007) Role of N–acetyl–seryl–aspartyl–lysyl–proline in the antifibrotic and anti-inflammatory effects of the angiotensin-converting enzyme inhibitor captopril in hypertension. Hypertension 49:695–703
Peng H, Carretero OA, Peterson EL, Rhaleb N-E (2010) Ac-SDKP inhibits transforming growth factor-beta1-induced differentiation of human cardiac fibroblasts into myofibroblasts. Am J Physiol Heart Circ Physiol 298:H1357–H1364
Peng H, Carretero OA, Raij L, Yang F, Kapke A, Rhaleb N-E (2001) Antifibrotic effects of N–acetyl–seryl–aspartyl–lysyl–proline on the heart and kidney in aldosterone-salt hypertensive rats. Hypertension 37:794–800
Pradelles P, Frobert Y, Creminon C, Ivonine H, Frindel E (1991) Distribution of a negative regulator of haematopoietic stem cell proliferation (AcSDKP) and thymosin beta 4 in mouse tissues. FEBS Lett 289:171–175
Pradelles P, Frobert Y, Creminon C, Liozon E, Masse A, Frindel E (1990) Negative regulator of pluripotent hematopoietic stem cell proliferation in human white blood cells and plasma as analysed by enzyme immunoassay. Biochem Biophys Res Commun 170:986–993
Rhaleb N-E, Peng H, Harding P, Tayeh M, LaPointe MC, Carretero OA (2001) Effect of N–acetyl–seryl–aspartyl–lysyl–proline on DNA and collagen synthesis in rat cardiac fibroblasts. Hypertension 37:827–832
Shi ZQ, Yu DH, Park M, Marshall M, Feng GS (2000) Molecular mechanism for the Shp-2 tyrosine phosphatase function in promoting growth factor stimulation of Erk activity. Mol Cell Biol 20:1526–1536
Sidhu JS, Omiecinski CJ (1997) An okadaic acid-sensitive pathway involved in the phenobarbital-mediated induction of CYP2B gene expression in primary rat hepatocyte cultures. J Pharmacol Exp Ther 282:1122–1129
Song M, Park JE, Park SG, Lee DH, Choi HK, Park BC, Ryu SE, Kim JH, Cho S (2009) NSC-87877, inhibitor of SHP-1/2 PTPs, inhibits dual-specificity phosphatase 26 (DUSP26). Biochem Biophys Res Commun 381:491–495
Sun H, Charles CH, Lau LF, Tonks NK (1993) MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell 75:487–493
Wang Y, Simonson MS, Pouyssegur J, Dunn MJ (1992) Endothelin rapidly stimulates mitogen-activated protein kinase activity in rat mesangial cells. Biochem J 287(Pt 2):589–594
Wang Z, Yang H, Tachado SD, Capo-Aponte JE, Bildin VN, Koziel H, Reinach PS (2006) Phosphatase-mediated crosstalk control of ERK and p38 MAPK signaling in corneal epithelial cells. Invest Ophthalmol Vis Sci 47:5267–5275
Warnecke C, Kaup D, Marienfeld U, Poller W, Yankah C, Grafe M, Fleck E, Regitz-Zagrosek V (2001) Adenovirus-mediated overexpression and stimulation of the human angiotensin II type 2 receptor in porcine cardiac fibroblasts does not modulate proliferation, collagen I mRNA expression and ERK1/ERK2 activity, but inhibits protein tyrosine phosphatases. J Mol Med 79:510–521
Wdzieczak-Bakala J, Fache MP, Lenfant M, Frindel E, Sainteny F (1990) AcSDKP, an inhibitor of CFU-S proliferation is synthesized in mice under steady-state conditions and secreted by bone marrow in long term culture. Leukemia (Baltimore) 4:235–237
Zhan Y, Counelis GJ, O'Rourke DM (2009) The protein tyrosine phosphatase SHP-2 is required for EGFRvIII oncogenic transformation in human glioblastoma cells. Exp Cell Res 315:2343–2357
Zhao Z, Tan Z, Diltz CD, You M, Fischer EH (1996) Activation of mitogen-activated protein (MAP) kinase pathway by pervanadate, a potent inhibitor of tyrosine phosphatases. J Biol Chem 271:22251–22255
Zhuo JL, Carretero OA, Peng H, Li XC, Regoli D, Neugebauer W, Rhaleb N-E (2007) Characterization and localization of Ac-SDKP receptor binding sites using 125I-labeled Hpp-Aca-SDKP in rat cardiac fibroblasts. Am J Physiol Heart Circ Physiol 292:H984–H993
Acknowledgments
This study was supported by National Institutes of Health grants HL 071806 (NER) and HL 028982 (OAC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peng, H., Carretero, O.A., Peterson, E.L. et al. N-Acetyl–seryl–aspartyl–lysyl–proline inhibits ET-1-induced collagen production by preserving Src homology 2-containing protein tyrosine phosphatase-2 activity in cardiac fibroblasts. Pflugers Arch - Eur J Physiol 464, 415–423 (2012). https://doi.org/10.1007/s00424-012-1150-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-012-1150-7