Abstract
Transepithelial Na+ transport is mediated by passive Na+ entry across the luminal membrane and exit through the basolateral membrane by two active mechanisms: the Na+/K+ pump and the second sodium pump. These processes are associated with the ouabain-sensitive Na+/K+-ATPase and the ouabain-insensitive, furosemide-inhibitable Na+-ATPase, respectively. Over the last 40 years, the second sodium pump has not been successfully associated with any particular membrane protein. Recently, however, purification and cloning of intestinal α-subunit of the Na+-ATPase from guinea pig allowed us to define it as a unique biochemical and molecular entity. The Na+- and Na+/K+-ATPase genes are at the same locus, atp1a1, but have independent promoters and some different exons. Herein, we spotlight the functional characteristics of the second sodium pump, and the associated Na+-ATPase, in the context of its role in transepithelial transport and its response to a variety of physiological and pathophysiological conditions. Identification of the Na+-ATPase gene (atna) allowed us, using a bioinformatics approach, to explore the tertiary structure of the protein in relation to other P-type ATPases and to predict regulatory sites in the promoter region. Potential regulatory sites linked to inflammation and cellular stress were identified in the atna gene. In addition, a human atna ortholog was recognized. Finally, experimental data obtained using spontaneously hypertensive rats suggest that the Na+-ATPase could play a role in the pathogenesis of essential hypertension. Thus, the participation of the second sodium pump in transepithelial Na+ transport and cellular Na+ homeostasis leads us to reconsider its role in health and disease.
Similar content being viewed by others
Introduction
The composition of body fluids depends on what is absorbed from the intestine and what is excreted in the sweat, breath, feces, and urine. The quantities and concentrations of electrolytes are mainly regulated by the kidney, since the intestine absorbs the vast majority of the water and salt that enter the intestinal lumen with no perceptible regulation.
Approximately 10 l of fluid pass through the intestine each day: 1.5–2.5 l is ingested, 1–1.5 l enters as saliva, 2–3 l is secreted in the stomach, and 1–2 l is secreted by the small intestine. Only 0.2 l is eliminated in the feces; the rest is reabsorbed in the small intestine and colon [93, 163]. The comportment of the intestine differs from one region to another. Thus, osmotic equilibrium is achieved in the proximal small intestine; net secretion occurs when the lumen of the proximal intestine contains a hypertonic fluid, whereas net absorption results when the content is hypotonic [56, 57]. There is net absorption of fluid and electrolytes from the resulting isotonic solution in the ileum and colon: The ileum absorbs approximately 9 l of isotonic fluid, whereas the colon absorbs 1 l of hypertonic solution. There is a distinct gradient of salt and water transport along the intestine. The unidirectional fluxes of salt and water in both directions are high in the duodenum and progressively diminish in the caudal direction. The fact that the absorptive flux decreases to a lesser extent than the secretory flux results in net absorption in the ileum and colon [175].
Epithelial Na+ transport
Under optimal conditions for transport, the proximal sections of the intestine absorb salt and water more rapidly than the distal segments, when expressed per unit length of intestine but not per unit mucosal surface. In addition, the pores across which diffusion takes place are probably larger in the proximal than in the distal region of the intestine (approximately 7.5 Å in the jejunum and 3.5 Å in the ileum). This feature restricts the passive movement of solutes in the distal gut so they exert greater osmotic pressure [93, 163].
The movement of ions and water from the intestinal lumen to the blood along the paracellular pathway occurs principally by passive diffusion as a result of electrochemical gradients and the Starling forces inherent in the vascular network. As far as the coupled movement of water and sodium is concerned, it has been proposed that water movement is passive and responds to the osmotic gradient created by the active transport of salt by the cells [48, 72, 88, 185].
In “leaky” epithelia (e.g., small intestine and proximal tubule) with high water permeability, the relationship between the absorption of sodium and water is such that the fluid absorbed is always isotonic sodium, and water can pass from the lumen to the blood by two different pathways, i.e., paracellular and transcellular. In this respect, the small intestine is classed as a “leaky” epithelium, characterized by a relatively small transepithelial electrical potential difference, very low electrical resistance and high permeability to small ions and water. This ensures that the fluids secreted and absorbed are isotonic. The passive permeability of the epithelium is, in fact, determined by the tight junctions.
Paracellular pathway
The paracellular pathway of the small intestine is extremely leaky to small ions, being only slightly selective for ions such as potassium. For instance, the permeability to K+ is about twice that to chloride, though the mobilities of these two ions in free solution are almost identical. In addition, there is relatively little discrimination between alkali metal ions. The relative permeabilities for Cs+/Rb+/K+/Na+/Cl−, determined in rabbit ileum, are 1.4:1.4:1.1:1.0:0.6. Moreover, the paracellular pathway is permeable to small molecules, such urea, arbinose, and xylose [82], and therefore, it behaves like an aqueous channel with a radius of 4.8 Å [50, 88, 149, 150, 152–154, 156, 175].
Transcellular pathway
Sodium enters the enterocyte across the apical pole of the cell and is then pumped into the lateral spaces by active processes located in the basolateral plasma membrane. The increased local osmotic pressure in the intercellular space causes water to leave the cell and also probably to pass from the lumen, across the tight junction, directly into the lateral spaces. The osmotic pressure is thereby reduced, but the hydrostatic pressure is increased, resulting in a movement of solvent towards the capillaries because of the high hydraulic conductance of these spaces. This process is aided by the colloid osmotic pressure of the plasma proteins, which contributes to the forces moving water into the capillaries. This flow of water also causes the phenomenon known as “solvent drag,” in which solutes are moved across the small intestine as a result of the interaction of small molecules with the fluid stream moving across the intercellular space [73, 82, 85, 86].
The transcellular movement of sodium is known to be dependent on cellular energy and to involve the participation of carriers. The sodium ion enters the enterocyte in at least three ways. First, there is an electrogenic movement of sodium ions across the apical pole of the cell with no direct coupling to the movement of other solutes; in this case, the sodium movement is associated with passive absorption of chloride. Second, the entry is coupled with that of a wide variety of non-electrolytes. Thirdly, there is a coupled movement of Na+ and Cl− across the brush-border membrane. Finally, sodium ions are actively extruded from the epithelial cells across the basolateral membrane against the electrochemical gradient.
Na+ entry to the intestinal epithelial cell
The electrogenic absorption of sodium
The cytoplasm maintains an electrical potential that is approximately 40 mV negative with respect to the solution bathing the mucosal face of the cell, and the intracellular sodium concentration is roughly one tenth of that in the mucosal and serosal fluids [5, 6, 81, 110, 152]. Sodium therefore enters the cell down an electrochemical gradient through sodium channels [60, 142, 146]. It is then expelled actively across the basolateral plasma membrane, as will be discussed in greater detail below. The active absorption of sodium is responsible for the maintenance of a small but significant transcellular potential difference of 3–5 mV (serosa positive), which drives the diffusional flux of chloride from mucosal to serosal fluid, either across the tight junctions or possibly also across the cell. In the mammalian intestine, sodium entry through Na+ channels is restricted to the colon [142, 146].
The coupled movement of sodium and organic solutes
The transport of a large range of water-soluble organic solutes, such hexoses, amino acids, vitamins, and bile salts, as well as diglycerides and triglycerides, depends on and is coupled to the absorption of sodium [14, 51, 59, 66, 147, 148, 152, 181, 182]. A ternary complex is formed in the brush-border membrane among a carrier, the substrate, and sodium ions, and this then crosses the membrane towards the interior of the cell as a result of the electrochemical gradient for sodium. Thus, the sodium gradient provides the energy necessary for the “uphill” transport of the solutes. These mechanisms were inferred from experiments with intact small intestine in vitro [151] and demonstrated unequivocally with the aid of brush-border membrane vesicles [75, 104, 144]. The sodium liberated into the cytoplasm is then pumped out of the cell actively across the basolateral plasma membrane, thus maintaining the low intracellular sodium concentration necessary for this type of transport on the one hand and the electrical force necessary for such a rheogenic process on the other [147]. The solute crosses the basolateral membrane by several mediated-transport mechanisms [14, 106, 183] from a region of higher concentration (the cytoplasm) to one of lower concentration (the basolateral space).
Neutral absorption of NaCl
Most of the sodium and chloride transport across the enterocyte entails an electroneutral process in which one sodium and one chloride ion are transferred in a coupled fashion. It was then suggested that the downhill movement of sodium would provide the energy necessary for the uphill transport of chloride ions, by direct analogy with the sodium-coupled transport of non-electrolytes. The chloride ion would leave the cell down an electrochemical gradient. An alternative model has been developed on the basis of experiments in vivo [170], brush-border membrane vesicles prepared from rat small intestine [29, 84, 105], and molecular characterization of the mechanisms [74, 94]. It has been suggested that the net NaCl absorption results from two processes in the apical membranes of the epithelial cells, exchange of Na+ and H+ and exchange of Cl− and HCO −3 . The bicarbonate and hydrogen ions are formed intracellularly from H2CO3 generated by the action of carbonic anhydrase, which is inhibited by acetazolamide. The downhill movement of sodium leads to a loss of H+ ions and therefore to an excess of base in the cytoplasm, which in turn leads to the downhill movement of bicarbonate in an outward direction and causes chloride to be accumulated, apparently against its electrochemical gradient. For this model to be valid, water and CO2 must be in thermodynamic equilibrium across the brush-border membrane. The two exchange systems must be interrelated and controlled by the intracellular pH. It is noteworthy that despite considerable efforts to locate a co-transport system for Na+ and Cl− in brush-border membrane vesicles of small intestine and proximal tubule, evidence for such as system has only been found in the dogfish rectal gland [55], the urinary bladder of the teleost winter flounder [135], and the distal convoluted tubule of the mammalian kidney [58].
Na+ extrusion across the basolateral plasma membrane of epithelial cells
Sodium ions are pumped out of the epithelial cells across the basolateral membrane against their electrochemical gradient by a process that requires energy. It has been demonstrated that this energy is derived from the hydrolysis of ATP and that at least one enzyme is responsible for such hydrolysis: the ubiquitous Na+/K+-ATPase, which has been identified in all animal cells. Numerous experiments are consistent with this notion. The cardiac glycoside ouabain only inhibits the active absorption of sodium when added to the serosal face of the tissue [147]. The inhibition of transepithelial sodium transport is accompanied by a loss in cell potassium and a gain in sodium. In addition, autoradiographic [49, 161], histochemical [52], immunohistochemical [1, 65, 100], and cell fraction studies [75, 129] have localized the binding of ouabain and the activity of the Na+/K+-ATPase almost exclusively to the basolateral cell membrane, with little or no activity in the apical pole of the epithelial cell. However, there is evidence that the intracellular Na+ concentration and water content are not tightly linked to the function of the Na+/K+ pump. Studies of uni- or bilateral exposure of rabbit ileal mucosa to a K+-free solution on the intracellular concentrations of cations and cellular water have provided the following results [108]: (a) removal of potassium from the mucosal surface has no effect; (b) bilateral removal of potassium causes a reduction in intracellular potassium and an equivalent gain in intracellular sodium, with no change in cell water; and (c) in contrast, removal of potassium from the serosal medium leads to a reduction in cell potassium without concomitant changes in sodium and or water contents. These observations suggest that the maintenance of the high intracellular potassium and low intracellular sodium concentrations depend on the presence of potassium at the serosal face of the cell and that the apical cell membrane is impermeable to potassium ions. The removal of sodium ions from the mucosal or serosal solutions leads to a fall in intracellular sodium levels but affects neither the intracellular potassium concentration nor the flux of potassium across the basolateral membrane; the bilateral removal of sodium causes a reduction in both intracellular sodium and potassium, a decrease in cell water and a diminution of potassium movement across the serosal membrane. In addition, ouabain reduces cell potassium and increases cell sodium by equivalent amounts without changing the cell water content. These various data support the hypothesis that the Na+/K+ exchange pump is responsible for maintaining the normal intracellular concentrations of sodium and potassium, but appear to indicate that the regulation of cell volume is independent of this process.
Additionally, there are several indications that the active transport of sodium across the intestinal epithelial cell is not uniquely dependent on a Na+/K+ exchange pump. Even when intracellular sodium is depleted and its transepithelial movement is abolished by removal of this cation from the mucosal face of the tissue, there is no change in either intracellular potassium concentration or cell water, and the trans-serosal flux of potassium is unaltered [108]. These observations must mean that the fluxes of sodium and potassium are not closely coupled and that neither trans-epithelial sodium transport nor the regulation of cell water is entirely dependent on the Na+/K+ exchange pump.
In addition, solutes such as d-glucose and l-alanine strongly enhance the transcellular movement of sodium by stimulating the entry of the cation across the apical pole of the cell [151]. However, these organic solutes do not influence the rate of exchange of 42K+ across the basolateral membrane [108]. These observations agree with the findings of Lee & Armstrong [81], who measured the intracellular activities of Na+ and K+ in bullfrog small intestine using cation-selective microelectrodes and observed that in the presence of 3-O-methyl-glucoside the ion activities were significantly reduced, despite the stimulation of transcellular sodium transport by this sugar. If there were an absolute relationship between the transport of sodium and the Na+/K+ exchange pump, an increase in cell potassium would be predicted. Indeed, these observations have been confirmed with isolated cells [155], where non-metabolizable hexoses elicited no rise in cell potassium. More recently, it has been proposed that changes in the rate of Na+ entry across the apical membrane, which should result in changes in the rate of basolateral membrane Na+/K+-pump activity and Na+ absorption, are accompanied by parallel changes in the K+ conductance across the basolateral membrane through K+ channels [68], avoiding the increase in intracellular potassium and hyperpolarizing the cell, which would induce Cl− exit. This KCl extrusion would permit the cell volume to be regulated. However, this hypothesis does not explain volume regulation in the presence of serosal ouabain.
The small intestine is not the only epithelium where there appears to be no strict relationship between transcellular sodium transport and sodium–potassium exchange, and indeed, findings of this nature were made early by numerous authors [11, 19, 30, 53, 54, 62, 91, 92, 137–139].
These observations suggest the existence of a second transport mechanism, independent of the Na+/K+-pump, which actively extrudes sodium across the basolateral plasma membrane of intestinal and renal epithelia.
Identification of a second sodium pump
In the proximal tubular cell of the guinea pig kidney, two different mechanisms for sodium transport across the basolateral membrane have been described and characterized [125, 126, 180]. One pump exchanges intracellular sodium for extracellular potassium, while the other actively expels sodium, passively followed by chloride ions and water. The former of these pumps is strongly inhibited by ouabain, weakly inhibited by ethacrynic acid and insensitive to furosemide and triflocin, whereas the second is refractory to ouabain but inhibited by ethacrynic acid, furosemide, and triflocin. Both processes are dependent on cellular energy since they are suppressed by 2,4-dinitrophenol or anoxia, indicating that they derive their energy from the hydrolysis of ATP. Similar mechanisms have been identified and characterized in isolated guinea pig small intestinal cells [46] and everted rat jejunum [167].
The enterocyte regulates its Na+ content by two pumps located in the basolateral plasma membrane. One exchanges Na+ for K+, is inhibited by ouabain, and insensitive to ethacrynic acid and furosemide. The second transports Na+ with Cl− and water, is insensitive to ouabain, but is inhibited by ethacrynic acid and furosemide. These results confirmed the evidence from experiments with inside–out basolateral plasma membrane vesicles from guinea pig small intestinal epithelial cells [43], rat jejunum [166] and rat proximal tubule [96], where two distinct mechanisms capable of accumulating sodium in the intravesicular space were demonstrated when ATP was added to the incubation medium. One transports sodium actively in the absence of potassium, whereas the other requires potassium to be present within the vesicles. The two mechanisms can also be differentiated by their affinities for sodium, their optimal pH, and their behavior towards different inhibitors. Thus, the active mechanism that transports sodium in the absence of potassium is refractory to ouabain but is inhibited by ethacrynic acid and furosemide, while the mechanism that causes sodium accumulation in the vesicles in the presence of internal potassium is strongly inhibited by ouabain, weakly inhibited by ethacrynic acid, and insensitive to furosemide. ATP is a specific stimulator of both processes and the requirement for magnesium is absolute in both cases.
These two active Na+ transport mechanisms, identified in epithelial cells of the small intestine and proximal tubule, are associated with ATPase activities located in the basolateral plasma membranes of such cells. The two Mg2+-dependent, sodium-stimulated ATPase activities have been identified in microsomal fractions [116] and crude basolateral plasma membrane fractions of the renal proximal tubule [45, 117] and purified basolateral plasma membranes of small intestinal cells [44]. In these preparations, the Na+-ATPase is stimulated by sodium alone or to a lesser extent by Li+, whereas the Na+-K+-ATPase requires both sodium and potassium for activation. These facts link the enzymes to the sodium transport systems. The Na+-ATPase specifically hydrolyzes ATP, as does the Na+-K+-ATPase, though the latter has some effect on GTP and ITP. This property defines the two enzymes as ATPases. The fact that the enzyme is stimulated indifferently by different sodium salts essentially excludes the possibility that the Na+-ATPase is an anion-stimulated ATPase, whose existence has been postulated [61]. The Na+-ATPase and the Na+-K+-ATPase can also be differentiated by their slightly different pH optima and different sensitivities to pH. They also reveal somewhat different affinities for sodium, the apparent K m values for sodium being 8–9 and 15–18 mM, respectively.
The two enzymes can also be distinguished by their different behavior towards a series of inhibitors. The Na+-ATPase is insensitive to ouabain but is inhibited by ethacrynic acid and furosemide and triflocin; in contrast, the Na+/K+-ATPase is fully inhibited by ouabain, partially inhibited by ethacrynic acid and unaffected by furosemide or triflocin. These features are of extreme importance, since they correspond exactly to the sensitivities of the two sodium-transporting mechanisms that have been characterized in renal [125, 180] and isolated small intestinal [46] cells. This correspondence provides the strongest evidence that each of the enzymes represents the machinery responsible for each one of the transport systems.
A model has been developed to explain the transepithelial transport of Na+ across the intestine (Fig. 1).
Theoretical model for transepithelial Na+-transport across the intestinal and renal epithelia. In the small intestine and renal proximal tubule, transepithelial Na+-transport depends on: (a) Na+ entry across the luminal membrane of the epithelial cells, following its electrochemical gradient and (b) Na+ extrusion through the basolateral plasma membrane by active mechanisms. The primary active Na+ transport has been mainly attributed to the Na+/K+ pump. However, two different mechanisms for active Na+ transport across the basolateral plasma membrane have been described in small intestinal and proximal tubular cells. One mechanism, corresponding to the Na+/K+ pump, requires K+, is inhibited by ouabain but is insensitive to furosemide. The other does not require K+, is insensitive to ouabain but inhibited by furosemide, and extrudes sodium with Cl− and water. This K+-independent, ouabain-insensitive mechanism had been denominated the second sodium pump and has been implicated in isosmotic cell volume regulation. These Na+ pumps have been associated with two different ATPase activities identified in the basolateral plasma membranes of enterocytes and proximal tubular epithelial cells. The Na+/K+ pump is linked to the Na+/K+-ATPase, which is Mg2+-dependent, stimulated by Na+ and K+, sensitive to ouabain and vanadate but insensitive to furosemide; while the K+-independent Na+ pump has been associated with the Na+-ATPase, which is Mg2+ dependent, specifically stimulated by Na+, does not require K+, and is insensitive to ouabain but inhibited by furosemide. This basolateral active Na+-transport, coupled to the passive Na+ entry through apical co-transporters, energizes the absorption of osmotically active solutes followed by water. This process generates isosmotic absorption of sodium, solutes and water
Identification of the ouabain-insensitive Na+-ATPase in different animal tissues
The ouabain-insensitive, Mg2+-dependent Na+-ATPase activity has also been identified in different animal tissues [102, 122]: arterial vascular muscle cells [115]; mammalian brain microsomal fractions [165]; sea bass (Dicentrarchus labrax L.) gills [12] and kidney [113]; squid gill microsomes [120]; shrimp (Macrobrachium amazonicum) gill homogenates [127]; gilthead bream (Sparus auratus L.) gills [173]; freshwater mussel (Anodonta cygnea) gills [77]; rainbow trout (Oncorhynchus mykiss Walbaum) gills [174]; rabbit cardiac sarcolemma [18]; malpighian tubules from Rhodnius prolixus [24, 25]; Trypanosoma cruzi epimastigotes [21, 145]; cultured MDCK I cells [39]; Entamoeba histolytica [38]; Leshmania amazonensis [36]; and pig kidney [79]. Recently, the Na+-ATPase activity has been reported in homogenates of several rat tissues [136].
The identification of an ouabain-insensitive Na+-ATPase in different animal species and tissues is very interesting because it suggests that the pump is universally distributed. However, the genes related to each of these enzymatic activities have to be characterized before the ubiquity of this ATPase can be accepted. For instance, the gene encoding the ouabain-insensitive Na+-ATPase in T. cruzi (TcENA or TrENA) [87] is different from that in mammals (atna) [140]. Alignment of atna and TcENA (by ClustalW) reveals that they encode different proteins. TcENA is much longer than ATNA. They only have 24 % identity, mainly related to the eight P-type ATPase motifs that they share. In addition, the binding site for the first cation has a significant modification. In fact, TcENA is a P-type ATPase more related to plant [158] or fungal [10] Na+-ATPases. Moreover, TcENA is functionally different from ATNA. TcENA is stimulated by Na+ and K+, while ATNA is specifically activated by Na+.
Modulation of the Na+-ATPase activity
The activity of the ouabain-insensitive, Mg2+-dependent Na+-ATPase can be modulated by several physiological conditions. Among the most relevant are:
Cell volume
Under isotonic conditions, there is a close relationship between the cell volume and the activity of the ouabain-insensitive Na+-pump, whereas the Na+/K+-pump activity is not affected by variations in cell volume [118]. The Na+-pump activity (Na+ transport and Na+-ATPase activity) is minimal when the cell water content is low but increases when the cell water content rises [124]. In addition, basolateral plasma membranes prepared from swollen proximal tubule cells of rat kidney show an ouabain-insensitive Na+-ATPase activity ten times higher than membranes isolated from control cells. If the swollen cells recover their volume, the activity decreases tenfold to control values.
High NaCl diet
High dietary NaCl intake induced an increase in the activity of the ouabain-insensitive Na+-ATPase. Healthy male rats exposed to chronic ingestion of isotonic NaCl solution for 4 months presented an increase (about 70 %) in the activity of the ouabain-insensitive Na+ pump in the basolateral plasma membranes of the kidney proximal tubular cells, whereas the ouabain-sensitive Na+/K+-pump activity did not change [95]. In addition, the ouabain-insensitive Na+-ATPase activity of kidney proximal tubular cells from rats fed with a high-Na+ diet for 4 months increased, while the Na+/K+-ATPase was not altered [111]. Moreover, proximal tubular kidney cells from rats chronically fed for 15 months with isotonic NaCl solution showed increases in kidney volume and in Na+ and Cl− content, as well as the activity of the ouabain-insensitive Na+-ATPase in the basolateral plasma membranes. These effects were reversed by returning the rats to drinking tap water. The authors propose that the Na+-ATPase activity is modulated in vivo by the cell volume [47].
Aging
The active Na+ transport mediated by the Na+/K+-pump and the active Na+-extrusion with Cl− and water through the second sodium pump were lower in old rats (24 months) than young ones (3 months). The oxygen consumption associated with each of the two active mechanisms of Na+ extrusion was also diminished in the old rats [123]. However, the turnover rate of the (Na+/K+)-ATPase was diminished by aging (about 40 %), while the Mg2+-dependent Na+-ATPase activity was similar in the kidneys of young and old rats, in both homogenates and basolateral plasma membrane fractions [97]. In contrast, it has been reported that the Na+- and Na+/K+-ATPases in jejunum epithelial cells have the same characteristics in the basolateral membrane of the enterocyte throughout the lifespan of the animal, but they quantitatively decrease with aging [168].
Angiotensins
Angiotensin II (Ang II) stimulates the Na+-ATPase activity in outer kidney cortex kidney [130], mediated by AT1 receptors through the PI-PLCβ/PKC pathway [40, 131–133]. Additionally, it has been shown, in inner kidney cortex, that Ang II inhibits the Na+-ATPase activity, mediated by AT2 receptors through a cholera toxin-sensitive PKA pathway [37]. These receptors are differentially distributed throughout the nephron, from outer to inner renal cortex, leading to a preferential binding of Ang II either to AT1 or AT2 receptors, respectively. Therefore, the predominant effect of Ang II on the Na+-ATPase in outer cortex would be stimulatory [40, 131–133], while in the inner cortex this peptide would have an inhibitory effect [37].
Ang-(1–7), as has been indicated for Ang II, has a dual effect on the Na+-ATPase. It selectively stimulates the enzyme in basolateral membranes of renal proximal tubules through AT1 receptors [23]. Moreover, experiments in which the AT1 receptors were blocked by losartan (an inhibitor of AT1 receptors) showed that Ang-(1–7) inhibits the proximal tubule Na+-ATPase by its interaction with AT2 receptors, which subsequently activate the Gi/o/cGMP/PKG pathway [35].
It is noteworthy that the stimulatory effect of Ang II in proximal tubule is reversed by Ang-(1–7) via Ang-(1–7)-specific receptors [78].
Nucleosides
Adenosine and inosine are purine nucleosides that modulate several physiological processes. Cellular signaling by adenosine occurs through four known receptor subtypes (A1, A2A, A2B, and A3). In the proximal tubule, adenosine decreases the activity of the ouabain-insensitive Na+-ATPase interacting with A1 subtype receptors through Gi protein pathway, without effect on the Na+/K+-ATPase [22]. Furthermore, in the presence of A1 selective antagonist, adenosine stimulates the Na+-ATPase, effect mediated by A2A receptors through PKA pathway [179].
Although the activation of PKA- or PKC-signaling pathways separately stimulates the Na+-ATPase activity [131, 179], the PKA pathway seems to be involved in a negative modulation of PKC-stimulatory effect when both ways are sequentially activated [63, 64]. Thus, the stimulatory effect of Ang II, mediated by PKC pathway, is reversed by adenosine through PKA pathway [63, 64]. In consequence, the existence of both stimulatory and inhibitory PKA-mediated phosphorylation sites in the Na-ATPase has been proposed [64]. The phosphorylation of the Na+-ATPase by PKC may induce a conformational change in the protein, which on turn might lead to exposure of inhibitory PKA-targeted sites. The phosphorylation of these inhibitory sites by PKA would reverse the stimulatory effect induced by PKC [64].
Inosine inhibits the renal ouabain-insensitive Na+-ATPase, an effect mediated by A1 receptor via Gi protein pathway [7].
Bradykinin
Bradykinin (BK), a peptide of nine amino acids, is a potent endothelium-dependent vasodilator that causes natriuresis. It has been reported that BK stimulates the ouabain-insensitive Na+-ATPase activity in kidney cortex homogenates (2.2 times) but inhibits the enzyme in basolateral membrane preparations by 60 %. The stimulation of the Na+-ATPase activity occurs through the interaction with B1 receptors, while the inhibitory effect on the enzyme is mediated through B2 receptors [27]. The effect of BK is mediated by activation of phosphoinositide-specific PLC β/PKC. The inhibitory effect is mediated by Ca2+-independent phospholipase A2, arachidonic acid (AA), and PGE2 [83], and seems to involve G-protein and PKA activation. Finally, it is interesting that BK counteracts the stimulatory effect of Ang-(1–7) on the proximal tubule Na+-ATPase activity through the B2 receptor [26, 89].
Purine bases
Adenine [177] and guanine [178] decrease the activity of the renal ouabain-insensitive Na+-ATPase through Gi protein-coupled receptors.
Urodilatin and atrial natriuretic peptide
Atrial natriuretic peptide (ANP) and urodilatin specifically inhibit the Na+-ATPase activity by activating the PKG pathway through the natriuretic peptide receptor (NPR-A) located in the luminal and basolateral membranes of proximal tubular cells [28, 176].
Epinephrine
It has been shown that norepinephrine stimulates the furosemide-sensitive Na+ pump and partially inhibits the ouabain-sensitive Na+/K+ pump, apparently through intracellular Ca2+ increase [119]. These effects are associated with both α- and β-adrenergic receptors [184]. In this sense, it has been shown that Ca2+ in the micromolar range stimulates the Na+-ATPase and partly inhibits the Na+/K+-ATPase of basolateral plasma membranes from guinea pig kidney [121], as well as the furosemide-sensitive ATP-induced Na+ transport in basolateral plasma membrane vesicles of rat kidney cortex [98], suggesting that Ca2+ could regulate the magnitude of Na+ extrusion with Cl− and water in proximal tubule epithelial cells.
Leptin, nitric oxide, ROS, and cyclic nucleotides
Chronic hyperleptinemia, induced by repeated subcutaneous leptin injections, increased cortical Na+/K+-ATPase, medullar Na+/K+-ATPase, and cortical Na+-ATPase [8]. This effect was prevented by co-administration of the superoxide dismutase mimetic tempol or the NADPH oxidase inhibitor apocynin. Acutely administered NO• donors decreased the Na+-ATPase activity. This effect was abolished by the soluble guanylate cyclase inhibitor ODQ (1H-[1, 2, 4]oxadiazolo[4,3,-a]quinoxalin-1-one), but not by PKG inhibitors. Exogenous cGMP reduced Na+-ATPase activity, but its synthetic analogues, 8-bromo-cGMP and 8-pCPT-cGMP, were ineffective. The inhibitory effect of NO• donors and cGMP was abolished by an inhibitor of cGMP-stimulated phosphodiesterase. An exogenous cAMP analogue and dibutyryl-cAMP increased the Na+-ATPase activity and abolished the inhibitory effect of cGMP. Finally, the administration of a superoxide-generating mixture (xanthine oxidase plus hypoxanthine) increased the Na+-ATPase activity. These results suggest that nitric oxide decreases renal Na+-ATPase activity by stimulating cGMP, which in turn activates PDE2 and decreases the cAMP concentration. Increased production of reactive oxygen species may lead to the stimulation of Na+-ATPase activity by scavenging NO• and limiting its inhibitory effect. The authors suggest that chronic hyperleptinemia is associated with an increase in Na+-ATPase activity due to excessive oxidative stress [9].
Lipid peroxidation and ethanol
It has been shown that lipid peroxidation [99] and ethanol [143] inhibit the Na+-ATPase.
Ceramide
Ceramide-activated PKA and PKC zeta inhibit the Na+-ATPase of the kidney proximal tubule [17].
Hypertension
The ouabain-insensitive Na+-ATPase activity and its regulation by Ang II in spontaneously hypertensive rats (SHR) has been evaluated [128]. Na+-ATPase activity was enhanced in 14-week-old but not 6-week-old SHR. The addition of Ang II decreased the enzyme activity in SHR to a level similar to that obtained in the Wistar–Kyoto rats used as controls. The inhibitory effect of Ang II was completely reversed by a specific antagonist of the AT2 receptor. Treatment of SHR with the AT1 receptor inhibitor losartan for 10 weeks (weeks 4–14) prevented the increase in Na+-ATPase activity observed in 14-week-old SHR. These results indicate a correlation between AT1-receptor activation and the increased ouabain-insensitive Na+-ATPase activity in SHR.
Our group has obtained evidence indicating that the Na+-ATPase activity is increased in basolateral plasma membranes of renal cortex from spontaneous hypertensive rats but not in the small intestine (Fig. 2a). Systemic treatment with Ang II increased the Na+-ATPase activity in both renal and small intestinal tissues (Fig. 2b). In agreement, the atna gene is overexpressed in renal cortex from SHR and Ang II-treated rats (Fig. 2c, d). These data suggest that the Na+-ATPase could be important in the pathogenesis of essential hypertension.
Regulation of the Na+-ATPase by angiotensin II and its possible role in arterial hypertension. a Na+- and Na+/K+-ATPase activities in renal cortex (upper graphic) and small intestine (lower graphic) from Wistar–Kyoto (control, open columns) or spontaneously hypertensive (SHR, closed columns) rats (200–250 g weight). Na+-ATPase activity is increased in the renal cortex of SHR. b Na+- and Na+/K+-ATPase activities in renal cortex (upper graphic) and small intestine (lower graphic) from Wistar–Kyoto rats treated (Ang II, closed columns) or not (control, open columns) with angiotensin II, administered through osmotic pumps at a dosage of 400 ng/kg body weight/day for 2 weeks. Na+-ATPase activity is increased in the renal cortex and small intestine of rats treated with angiotensin II. c, d Semi-quantitative expression level of Na+-ATPase mRNA (atna) in different rat specimens. Total RNA from untreated Wistar–Kyoto (control, specimens C1 and C2), Wistar–Kyoto treated with angiotensin II (specimens A1 and A2) and SHR was isolated using Trizol reagent and following the manufacturer’s indications. The first cDNA strand was synthesized through the Thermoscript RT-PCR system, using Oligo(dT)20 as primer. The PCR step was carried out with specific primers for Na+-ATPase or β-actin cDNA (house-keeping gene), as described [140]. Electrophoretic analysis of the PCR product on 2 % agarose gels (c) and the respective densitometric quantification (d) are shown. Bars represent the relative Na+-ATPase mRNA expression normalized to the β-actin house-keeping gene as arbitrary units. The Na+-ATPase mRNA is over-expressed in rats treated with angiotensin II and in SHR
The multiple modulation of the activity of the Na+-ATPase suggests the relevance of this enzyme to renal and intestinal sodium homeostasis.
Isolation and characterization of the intestinal ouabain-insensitive Na+-ATPase
Despite the extensive biochemical, functional, and pharmacological evidence indicating the existence and the physiological relevance of the ouabain-sensitive Na+-ATPase in different tissues, no particular protein or gene related to ATPase activity had been identified until recently. Our group has been able to solubilize both the Na+- and Na+/K+-ATPases from the enterocyte basolateral plasma membrane without inactivation, to separate them physically using Con-A affinity chromatography and to purify the Na+-ATPase by anion-exchange chromatography [140]. The purified enzyme retains the functional characteristics of the native enzyme, e.g., Mg2+ dependence, specific stimulation by sodium, insensitivity to ouabain, and inhibition by furosemide and vanadate. Electrophoretic analysis and anion-exchange chromatography demonstrate that the Na+-ATPase is a protein complex comprising at least two subunits of 90 kDa (α-subunit) and 50 kDa (β-subunit). The 50 kDa subunit is glycosylated and could be a previously undescribed P-type ATPase β-subunit. Although the available sequence evidence is not conclusive, its N-terminal sequence (SPLEYQD) does not correspond to any previously reported β-subunit [140].
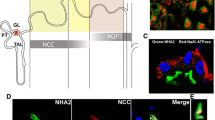
As shown in Fig. 3, the distribution of the Na+- and Na+/K+-ATPase differs through distinct guinea pig kidney segments. Both enzymes are well expressed in the outer cortex, but Na+-ATPase expression is lower in the inner regions of the kidney and absent in the medulla. In the intestine, the Na+-ATPase is mainly expressed in villous (small intestine) and surface (colon) cells. In the crypt region (immature cells), the enzyme seems to have an intracellular distribution. This particular renal and intestinal distribution probably has to do with the physiological role of this enzyme in sodium transport in these epithelia. Furthermore, IgY polyclonal antibodies raised against the purified Na+- and Na+/K+-ATPases differentially recognize these enzymes. Antibodies raised against the purified Na+-ATPase inhibit the Mg2+-dependent ouabain-insensitive Na+-stimulated ATPase activity without effect on the Na+/K+-ATPase, while antibodies raised against the purified Na+/K+-ATPase inhibit this enzyme without effect on the Na+-ATPase (Fig. 4).
Immuno-localization of the Na+- and Na+/K+-ATPases in intestinal and renal tissues. Kidney and small intestine (jejunum) were obtained from guinea pig (Cavia porcellus), embedded in Cryomatrix, frozen in liquid nitrogen and sectioned (8 μm) in a cryostat microtome at −20°C and mounted on glass slides coated with poly-l-lysine. Sections were fixed in acetone at −20°C, dried in air, and treated for immune-fluorescence or immune-histochemistry. a Immunolocalization of Na+- and Na+/K+-ATPases in different kidney slices, as indicated (outer cortex and medulla, inner cortex, and medulla). Na+-ATPase was detected using its IgY polyclonal antibodies and a goat polyclonal anti-chicken IgG labeled with tetramethyl-rhodamine isothiocyanate (red), while Na+/K+-ATPase was identified using a horse anti-Na+/K+-ATPase polyclonal antibody and a rabbit polyclonal anti-horse IgG labeled with fluorescein isothiocyanate (green). b Immunohistochemistry of guinea pig jejunum using anti-Na+/K+-ATPase (a, c, e) or anti-Na+-ATPase (b, d, f) IgY polyclonal antibodies. Sections were incubated with a rabbit polyclonal anti-chicken-IgY conjugated with peroxidase (1:1,000 dilution in PBS). Note the differential distribution of Na+- and Na+/K+- ATPases in both renal and intestinal tissues
Effect of anti-Na+-ATPase and anti-Na+/K+-ATPase IgY polyclonal antibodies on sodium ATPase activities. Basolateral plasma membranes from small intestine, obtained as described [41], were pre-incubated with pre-immune IgY (control, open bars) or anti-Na+-ATPase IgY polyclonal antibodies (anti-Na+-ATPase, ruled bars) or anti-Na+/K+-ATPase IgY polyclonal antibodies (anti-Na+/K+-ATPase, closed bars) in a 1:500 dilution for 30 min at 4°C. Each antibody specifically inhibited the ATPase from which it was developed
Na+-ATPase forms a phosphorylated intermediate
The Na+-ATPase can be classified among the P-type ATPases. Its Mg2+ dependence, sensitivity to vanadate and capacity to form a phosphorylated intermediate from ATP or Pi are the strongest pieces of evidence for this classification. It can be phosphorylated from inorganic phosphate in an ion-sensitive reaction stabilized by furosemide [164]. In that article, a phosphorylated polypeptide of about 100 kDa was identified for the first time as directly associated with the Na+-ATPase. In 2005, del Castillo et al. [42] reported a phosphorylated intermediate obtained from [32P]-ATP associated with the purified Na+-ATPase. The phosphorylation was Mg2+ dependent, vanadate-sensitive and stimulated by Na+ with two different K m values (0.66 and 15 mM). The stimulatory effect was specific for Na+ and independent of anions. Phosphorylation was insensitive to ouabain but stimulated by furosemide with an EC50 of 1.8 ± 0.54 mM. In addition, 0.5 mM ADP partially (50 %) inhibited it. Phosphorylation was also sensitive to alkaline pH and hydroxylamine, suggesting an acyl-phosphate bond associated with the 100 kDa polypeptide of the enzyme.
A minimum reaction cycle for the Na+-ATPase was proposed in which the enzyme has an E1 form that can be phosphorylated from ATP in the presence of Mg2+ and Na+, producing the E1.P.Na form, sensitive to ADP. Furosemide stabilizes the E1.P.Na form. The enzyme then changes to the E2.P.Na form, insensitive to ADP, which is susceptible to dephosphorylation. A conformational change induces Na+ translocation through the membrane. Later, a phosphorylated intermediate associated with the ouabain-insensitive Na+-ATPase was identified by De Souza et al. [39] in microsomal fractions of cultured MDCK I cells and by Ventrella et al. 2010 [172] in homogenate fractions of rat kidney and microsomal fractions of rainbow trout gills. Both articles have several discrepancies, but the most important is that furosemide totally inhibits the Na+-stimulated phosphorylation in MDCK cells but enhances phosphorylation in rat kidney and trout gills. The data emerging from these studies, which used homogenates or microsomal fractions in which different ATPase and phosphatase activities coexist, are very difficult to interpret. However, the results obtained with the purified Na+-ATPase demonstrated that furosemide stabilizes the phosphorylated intermediate in an E1.P.Na form, sensitive to ADP, increasing the observed phosphorylation [45].
Cloning of the ouabain-insensitive Na+-ATPase (atna cDNA)
The atna complementary DNA (cDNA) that codes for the ouabain-insensitive, K+-independent, Na+-ATPase was recently cloned from guinea pig intestinal epithelial cells (EF489487.2, GI:283442232) [140]. It was amplified by two strategies based on degenerate PCR.
The first approach was based on the use of degenerate primers designed from consensus sequences for the two best-conserved P-type ATPase structural motifs (the phosphorylation and nucleotide-binding motifs), since the ouabain-insensitive Na+-ATPase has features of this protein family. This strategy allowed seven P-type ATPase cDNAs to be cloned, which belonged to subtypes P2A (SERCA2 and SERCA3), P2B (PMCA1 and PMCA4), and P2C (AT12A, AT1A1 and ATNA) [141]. They included a new ATPase cDNA fragment of 902 bp, strongly related to atp1a1, which was named atna.
The second strategy was based on successive reverse transcription PCR (RT-PCR) and hemi-nested PCR, which employed primers targeted to the three peptides identified by tandem-mass spectrometry of the purified ouabain-insensitive Na+-ATPase [140]. Interestingly, these three peptides are shared by the α-subunit of the Na+- and Na+/K+-ATPases. As expected, when this strategy was applied, two different cDNA fragments were cloned: one fragment corresponded to the α1-isoform of Na+/K+-ATPase (AT1A1) and the other matched with the atna fragment, cloned in the first strategy.
The sequence of guinea pig atna cDNA was completed by RLM-RACE for 5′- and 3′ ends. It has 2,787 nucleotides that include the following: (a) the 5′-untranslated region (5′-UTR) of 163 residues that begins with adenosine; (b) an open reading frame (ORF) of 2,436 bases that encodes a protein with 811 amino acids; and (c) a 3′-untranslated region (3′-UTR) 188 bases long in which the polyA-signal and polyA-site, necessary for messenger RNA (mRNA) maturation, were identified [140]. It was demonstrated that this cDNA codes for the ouabain-insensitive Na+-ATPase through silencing experiments in MDCK cells, a dog kidney cellular lineage that express a K+-independent, ouabain-insensitive Na+-ATPase [39, 140]. The atna cDNA was cloned from MDCK cells, employing the second strategy applied in guinea pig. A specific small-interfering RNA was designed from this cDNA sequence, and interference experiments were performed in MDCK cells. The silencing of the atna cDNA specifically inhibited both the ouabain-insensitive Na+-ATPase activity and the expression of its α-subunit [140].
Structural analysis of ATNA protein
The ATNA-encoded protein has 811 amino acids with a probable molecular weight of 88,940 Da and an estimated pI of 5.70. As shown in Fig. 5a, the amino acid sequence of the ATNA protein has all P-type ATPases structural motifs described for this protein family [76, 101, 114], including the P-type ATPase signature-motif “DKTGT(L/I)T,” the dehalogenase-motif (GDGVND) and the phosphatase-motif (“TGE”).
Structural features of ATNA protein. a Analysis of ATNA amino acid sequence. Primary structure of guinea pig ATNA (ATNA_CAVPO: EF489487.2) consisting of 811 amino acid residues is shown. Transmembrane segments (M1–M6), predicted by ClustalW [80] alignment homology and analyzed through TMpred and MPEx [160], are enclosed in squares. P-type ATPase structural motifs are emphasized in bold. The protein segment included in the 3D-prediction analysis (shown below in b) black letters. b Predicted 3D-structure of ATNA. Three-dimensional structure of ATNA_CAVPO was iteratively modeled with the non-redundant PDB database by CPHmodels 3.0 [109]. This program aligned the query amino acid sequences with a segment of the rat Na+/K+-ATPase (AT1A1_RAT: P06685), employed as template, whose partial crystalline structure had been previously determined in the E2-P conformation [69]. A lateral view of the protein is shown, showing P-type ATPase structural domains, active site and some relevant amino acid residues (Glu-322, Ile-724, and Asp-754). Only the relevant residues are shown in ball and stick representation. The amino acids Glu-322 and Asp-754 conform to the cation-binding sites I and II. c 3D structure of ATNA active site. A close-up of the ATNA_CAVPO active site is shown from the cytosolic perspective employing the three-dimensional structure obtained in b. Only the relevant amino acid residues are shown in ball and stick representation. The phosphorylatable Asp-364 is highlighted in yellow. The conformation of the nucleotide binding (Thr-560, Gly-561, Lys-641, Asp-660, and Asn-661), kinase (Asp-364, Lys-365, and Thr-366) and phosphatase (Thr-207, Gly-208, and Glu-209) domains are indicated. The cation-binding site I (Glu-322) is also shown
The amino acid residues considered essential for P-type ATPase function [15, 103] seem to be present in ATNA. Sequence alignment through ClustalW [80] (Fig. 6a) and three-dimensional topology prediction by CPHmodels 3.0 program [109] (Fig. 5b, c) allow the homologous residues at the corresponding positions described for AT1A1_PIG and SERCA1_RABIT ATPases, whose crystalline structure was previously elucidated [112, 157, 169], to be identified in ATNA. The homology comparison is summarized in Table 1. In fact, all essential residues are identical in ATNA and AT1A1 and differ in only one position from SERCA1 (Asp-754 changes to Asn-796 in SERCA1). Although it is reasonable to suppose that homologous residues play similar functions, this requires experimental demonstration. Nevertheless, homology analysis strongly suggests that ATNA_CAVPO has the amino acid residues essential for ATP hydrolysis (Fig. 5c) [15, 103], including the phosphorylatable amino acid (Asp-364) and the residues necessary for nucleotide binding (Thr-560, Gly-561, Lys-641, Asp-660, and Asn-663), enzyme phosphorylation (Asp-364, Lys-365, and Thr-366 from the kinase domain) and enzyme dephosphorylation (residues Thr-207, Gly-208, and Glu-209 from the phosphatase domain).
Comparative analysis of ATNA and AT1A1. a Alignment of ATNA and AT1A1 amino acid sequences. The amino acid sequences of ATNA_CAVPO (EF489487.2) and AT1A1_CAVPO (EF489488.2) were aligned through ClustalW 2.1 [80] with the default setting. The classic alignment style is shown: identical residues in red (asterisk), high-similarity residues in green (colon), low-similarity residues in blue (period), and different residues in black. P-type ATPase structural motifs are emphasized in bold. Transmembrane segments, predicted by homology, are highlighted with yellow shadow. The region (45CKR) of 45 amino acids, present in all cation/K+-ATPases but absent in the ATNA protein, is emphasized with gray shadow. b Three-dimensional comparison between ATNA and AT1A1 from guinea pig. Predicted 3D structures of ATNA_CAVPO and AT1A1_CAVPO were iteratively modeled with the non-redundant PDB database by CPHmodels 3.0 [109] (as described in Fig. 5b). Lateral views of both proteins are shown, revealing P-type ATPase structural domains. The atoms of some relevant amino acid residues are represented as space fill. The location of 45CKR in the AT1A1_CAVPO structure is indicated with standard chemical element colors. The neighbor amino acid pair delimiting the 45CKR in AT1A1_CAVPO (Lys-445 and Leu-491) and the homologous amino acid residues of ATNA_CAVPO are marked in orange. c Chemical interaction between AT1A1 and its inhibitor ouabain. The crystallized 3D structure of the Na+/K+-ATPase holoenzyme from pig is shown in E2-P conformation with ouabain-bound (PDB: 3N23). The Na+/K+-ATPase alpha, beta and gamma chains are colored blue, orange, and green, respectively. Ouabain is marked in red. The atoms of ouabain and the amino acid residues in the M1 and EC1 segments of AT1A1_CAVPO are represented as space fill. Two views are shown: lateral (left) and extracellular (right) after 90° lateral rotation
Additionally, TMpred and MPEx [160] programs predict at least six transmembrane α-helices (M1–M6) that match the P-type ATPase core-protein, which is considered to be constituted by four characteristic domains: nucleotide binding, kinase, phosphatase, and transmembrane [90]. In this sense, the segments M1, M2, M4, M5, and M6 seem to form the half-channels for Na+ transport. Moreover, ATNA seems to have the essential amino acid residues for cation transport, following a model of alternating access [103] without counter-ions. The relevant residues for this model include Glut-322, Ser-725, Asn-726, Glu-729, Asp-754, and Asp758. The residues Glut-322 and Asp-754, respectively, located in M4 and M6, seem to be involved in Na+ binding. Thus, Glu-322 would constitute the Na+-binding site I, while site II should be simultaneously formed by Glu-322 plus Asp-804 [4, 15, 32, 103]. Additional residues such as Ser-725, Asn-726, Glu-729, and Asp758 may also participate in cation coordination. The segment M1 of ATNA has the residue Leu-98, which would function as the cation gate-lock for Na+ occlusion after enzyme phosphorylation and during the E1P-E2P transition [15, 103]. Therefore, ATNA could pump one or two Na+ ions per catalytic cycle. These analyses suggest that ATNA has the necessary elements to couple the exergonic hydrolysis of ATP with the endergonic transport of Na+ against its electrochemical gradient.
Comparison between Na+- and Na+/K+-ATPases (ATNA and AT1A1 proteins)
ATNA protein has 64 % identity and 72 % high similarity to AT1A1 protein from guinea pig [140]. However, the differences are not uniformly distributed along their primary structures but grouped as clusters at the amino and carboxyl terminal ends (Fig. 6a). In addition, ATNA lacks a region of 45 amino acids (45CKR) present in the nucleotide-binding domain of all cation/K+-ATPases, including AT1A1 [140, 141].
Some features could explain the functional differences observed between the K+-independent, ouabain-insensitive Na+-ATPase (ATNA) and the Na+/K+-ATPase (AT1A1). The three-dimensional structure prediction using CPHmodels 3.0 [109] shows that 45CKR is located between the phosphatase domain and the phosphorylation site in the E2P conformation. In the cation/K+-ATPases, we have proposed [140] that the 45CKR could prevent the approximation of the phosphatase domain to the phosphoryl-aspartate, stabilizing the phosphoryl-enzyme in its E2P conformation until K+ is bound. Once K+ is bound, it should induce an additional conformational change that permits the phosphatase domain to interact with the aspartyl-phosphate and the subsequent dephosphorylation of the enzyme. The absence of 45CKR in ATNA could permit direct dephosphorylation, without K+ binding, which would explain the K+-independence of ATNA.
Two structural characteristic of ATNA in M1-EC1 and M5 could explain its ouabain resistance. The segments M1 and EC1 of the Na+/K+-ATPase α-subunit have been implicated in ouabain binding [112, 157]. Figure 6c shows the pig Na+/K+-ATPase holoenzyme bound to ouabain (red space-filling atoms), which interacts closely with the M1 and EC1 segments of the enzyme (blue space-filling atoms). These segments show important modifications in the amino acid sequence of ATNA (Fig. 6a) that could preclude ouabain binding. Moreover, the residue Thr-774, present in M5 of AT1A1 (Fig. 6c), is replaced by Ile-724 in ATNA_CAVPO (Fig. 6a). It has been shown that the single substitution of Thr-774 by alanine transforms the Na+/K+-ATPase in an ouabain-insensitive enzyme [70]. Thus, the replacement of this threonine residue by isoleucine (Ile-724) in ATNA could result in the characteristic ouabain-insensitivity of this enzyme.
Phylogenetic analysis of ATNA
Sequence alignment of 78 P-type ATPases of all sub-types described was performed by ClustalW 2.1 [2] and the un-rooted dendrogram was drawn with Unrooted.exe. The resulting phylogenetic tree is shown in Fig. 7a. As expected, ten clearly defined branches were identified, corresponding to the ten described sub-types. These results situate ATNA in sub-type IIC (P2c), which includes the four α-isoforms of the Na+/K+-ATPase (AT1A1-4), the two α-isoforms of the H+/K+-ATPase (ATP4A and ATP12A), the invertebrate α-subunit of Na+/K+-ATPase, and the Na+-ATPase from the alga Heterosigma akashiwo.
Phylogenetic analysis of ATNA. a Phylogenetic tree of P-type ATPase family. A total of 78 amino acid sequences of P-type ATPases, representing the ten sub-types described (P1A-P5), as indicated, were aligned through ClustalW 2.1 [80]. The transported cations are indicated with colors: K+ (yellow), heavy metals (brown), Ca2+ (hot pink), Na+ (blue), Na+ and K+ (light green), H+ and K+ (salmon), H+ (orange), Mg2+ (purple), amino-phospholipids (dark green), and unknown cations (gray). The clue numbers and their respective Swiss-prot access numbers of the aligned sequences are 1:P03960, 2:Q8Z8E5, 3:Q04656, 4:P70705, 5:Q64446, 6:Q9XT50, 7:Q9M3H5, 8:Q9S7J8, 9:Q59465, 10:O08462, 11:Q59207; 12:P13585, 13:P70083, 14:Q03669, 15:Q00779, 16:Q9YGL9, 17:Q93084, 18:P57709, 19:Q80XR2, 20:Q00804, 21:P11505, 22:Q9R0K7, 23:P58165, 24:Q16720, 25:Q64568, 26:P23634, 27:Q64542, 28:P38929, 29:Q37145, 30:P09572, 31:(GI:EF489488.2), 32:P50993, 33:P06686, 34:P13637, 35:P58312, 36:Q13733, 37:Q64541, 38:P05025, 39:P28774, 40:P13607, 41:P35317, 42:P50996, 43:Q92126, 44:Q92036, 45:Q64392, 46:(GI: EF489487.2), 47:Q9SXK5, 48:P13587, 49:Q01896, 50:Q12691, 51:P22189, 52:Q58623, 53:P11718, 54:P12522, 55:P20649, 56:P09627, 57:P0ABB8, 58:P0ABB9, 59:P36640, 60:P22036, 61:O54827, 62:O94823, 63:P98197, 64:Q9N0Z4, 65:Q8NB49, 66:Q29449, 67:P98200, 68:O43520, 69:P98204, 70:O70228, 71:O43861, 72:P39524, 73:Q9CTG6, 74:Q9HD20, 75:Q9H7F0, 76:O14072, 77:O74431, and 78:Q9LT02. The putative P-type ATPase phylogenetic precursor (Q58378) from Methanococcus jannaschii was included in the alignment (white circle, clue number: 0). Unrooted dendrograms were drawn with Unrooted.exe using the ClustalW alignment data. Defined phylogenetic branches are shadowed in gray and ATPase sub-types are indicated. b Phylogenetic tree of sodium-stimulated P-type ATPases. ClustalW aligment and Unrooted dendrogram drawn was repeated with only sodium-stimulated P-type ATPases (clue numbers 30–41 and 46–50). Defined phylogenetic branches are shadowed in gray and taxonomic lineages are indicated. The transported cations are indicated with colors: Na+ (blue) or Na+ plus K+ (light green). PMCA plasma membrane Ca2+-ATPases, SERCA sarcoplasmic/endoplasmic reticulum Ca2+-ATPases, PMR1-2 Golgi apparatus membrane Ca2+-ATPases, ATNA guinea pig ouabain-insensitive Na+-ATPase. The α–isoforms of the Na+/K+-ATPase are indicated
The sequence alignment of only Na+-stimulated ATPases identified four branches, each corresponding to a single taxonomic lineage: yeast, alga, invertebrate, and vertebrate (Fig. 7b). As expected, the vertebrate and invertebrate ATPases are more closely phylogenetically related. For example, the Na+/K+-ATPases from the vertebrate Torpedo californica (SP:P05025, clue number 38), and the invertebrate Artemia sanfranciscana (SP:P28774, clue number 39) are 77 % identical. In contrast, the artemia ATPase is only 26 % identical to the Na+-ATPase from Saccharomyces cerevisiae (SP:Q01896, clue number 50). This suggests that Na+-stimulated ATPases from vertebrates and invertebrates have diverged recently, conforming with close phylogenetic groups in the animal kingdom, and their Na+ dependence seems to have originated independently of similar Na+-ATPases from plants and fungi.
ATNA shares the highest identity with AT1A1 (64 %) in guinea pig. Homology with other Na+-ATPases, with unknown or doubtful K+ dependence, is lower. Thus, ATNA is 56 % identical to the Na+-ATPase from the alga H. akashiwo (SP:Q9SXK5, clue number 47) and 24–43 % identical to some yeast Na+-ATPases (SP:P13587, Q01896, and Q12691, clue numbers 48–50, respectively). These results agree with the evidence that atna and atp1a1 share the same locus (atp1a1) and did not follow independent evolutionary pathways since they are still inseparable genes. In fact, both transcripts share exons.
The origin of atna seems to precede the divergence of mammals from the rest of the vertebrates since it is broadly spread in this taxonomic class. The atna gene could have been generated from insertional events plus the duplication of some atp1a1 exons, which followed divergent evolution.
The ouabain-insensitive Na+-ATPase (atna) gene in guinea pig and human
The search for the atna gene in the guinea pig genomic database reveals that atna and the Na+/K+-ATPase α1-isoform (atp1a1) cDNAs are at the same genetic locus: atp1a1. The atna mRNA shares 13 exons with atp1a1 mRNA, but has five exclusive exons located at the 5′ and 3′ ends. The transcription start sites (TSS) for both transcripts are separated by more than 8.4 kb, suggesting that these mRNAs are independently transcribed from independent promoters. The programs Promoter Scan and TFsearch predict one putative atna promoter downstream of the atp1a1 promoter. The guinea pig atna promoter (Fig. 8a) includes the following: (a) TATA-box, (b) two overlapping initiators (Lyf-1 and Ik2), and (c) four HSF sites [140]. These features are likely to be enough to allow the atna gene to be independently transcribed, as described for other genes [13, 16].
Search for atna gene in human and guinea pig. a Location of atna gene in genomic DNA of human and guinea pig. The human gene was identified by alignment of the amino acid sequence of ATNA_CAVPO (EF489487.2) with the human genomic DNA around locus atp1a1 (chromosome 1, short arm, GRCh37, plus strand), translated in the three frames for both strands, employing NCBI Blast5 bl2seq TBLASTN [2]. Exon–intron distribution was defined through NCBI Spidey by alignment between the 2787-bp guinea pig atna mRNA (EF489487.2) and the respective genomic DNA segment for human or guinea pig. For human (top), Homo sapiens atp1a1 locus from base +116925339 to base +116952443 was aligned. For guinea pig (bottom), Cavia porcellus atp1a1 locus from base +40790086 to base +40813062 (scaffold 2, plus strand) was used. It allowed all exons and introns to be identified, including the ending exons. Analysis of results is shown as a proportional uni-dimensional scheme of exon/intron distribution, where exons (pink or red boxes) and introns (blue connector line) are represented at scaled width. Identification of atna promoters for human (b) and guinea pig (c). Once the first exon was identified, promoters were identified through BDGP Neural Network Promoter Prediction [134]. Response elements for transcriptional factors were predicted by TFSearch [67]. Transcriptional start site (TSS) is defined on +1. Promoters, initiators, transcriptional factor elements, start codons and splice donor sites are identified, enclosed in squares. The predicted transcribed segment is shown shadowed. First exon and first intron are shadowed yellow and gray, respectively. Encoded amino acid sequence for first exon is shown below in bold script
Given that the ouabain-insensitive Na+-ATPase has been described in other species, as indicated above, we decided to explore the existence of a putative orthologous gene in humans. Therefore, the human genome from the ENSEMBL database was analyzed with TBLASTN [2], using the ATNA amino acid sequence as input (Fig. 8b, top). The atna gene seems to be located in the plus strand of the locus atp1a1, in the short arm of chromosome 1, near to the centromere (locus GRCh37 from +116915290 to +116952883). The start codon appears to be encoded by the human genomic nucleotides +116925339 to +116925341.
The exon–intron distributions for guinea pig and human were determined using the program NCBI Spidey by aligning the 2,787-bp guinea pig atna mRNA (EF489487.2) and the respective genomic DNA segment. All exons of the atna ortholog from human were identified. The exon–intron arrangements for atna in the atp1a1 loci from human (top) and guinea pig (bottom) are shown in Fig. 8b. The human atna seems to have 21 or 22 exons (one exon is very short) located in the atp1a1 locus from base +116925339 to +116952443, showing an exon–intron pattern similar to the guinea pig gene.
Once the human first exon was located, the promoter was identified using the program BDGP Neural Network Promoter Prediction [134], and Response Elements for Transcriptional factors were predicted by TFSearch [67]. The TSS, represented by an adenosine residue, corresponds to base +116924704 (chromosome 1), located 164 bases upstream of the putative start codon (Fig. 8c). This predicted promoter, as in the guinea pig ortholog (Fig. 8a), includes a TATA-box, a GC-rich box and two initiators Lyf-1 and Ik2. These initiators have been involved in immune cell differentiation [71] and the inflammatory response, as negative regulators of iNOS [31] and upregulators of IL10 expression [171]. In addition, the human promoter region has another putative initiator element, 87 bases downstream of the described TSS [34, 159].
Heat-shock-factor elements (HSE) are genetic sequences located in promoter regions, recognized by heat-shock transcriptional factors (HSF), regulatory proteins that modulate gene expression [3]. It is noteworthy that the four putative HSE sites present in the atna promoter (from −249 to −76) are absent in the atp1a1 gene. This opens the possibility that the expression of these two genes could be differentially regulated in response to physiological or stress situations (e.g., hormone stimulation, heat shock, nutritional starvation, and osmotic stress). HSF-1 is activated by osmotic stress, inducing several genes [20]. If HSE in the atna gene responds to HSF1, its overexpression would allow the cell to extract osmotic particles such as Na+ ions to compensate the osmotic disturbance. It is interesting that other inducers of HSF-1, such as ethanol, cell volume alteration, oxidative stress, and nutritional stress, modulate the Na+-ATPase activity, as mentioned above.
The presence of predicted response elements for HSF, Lyf-1, and Ik2 in the atna promoter allows us to hypothesize that atna could participate in the epithelial inflammatory response. It has been shown that HSF-1, activated in febrile states, can also modify the expression of non-HSP genes including those for cytokines and chemokines [33, 107]. Moreover, Tanaka and Mizushima [162] showed that the activation of HSF-1 protects against both irritant-induced gastric lesions and IBD-related colitis, promoting tissue repair.
Why do cells express the K+-independent Na+-ATPase and the Na+/K+-ATPase with apparent overlapping functions in active sodium extrusion?
The identification of the atna gene and its encoded protein, the K+-independent, ouabain-insensitive Na+-ATPase, represents a breakthrough in the understanding of epithelial Na+ transport. It provides exceptional biochemical and molecular evidence to explain the multiple functional data that suggested the existence of an active Na+ transport, independent of the Na+/K+ pump, in renal and intestinal epithelia. The presence of the second sodium pump in the basolateral plasma membrane would allow the epithelial cells to extrude Na+, Cl−, and water under circumstances where transepithelial Na+ transport is highly stimulated (i.e., transepithelial Na transport coupled to sugars and amino acids), with no relevant effect on the activity of the Na+-K+ exchange pump. Under these conditions, the electroneutral movement of Na+ and Cl− by the second sodium pump would eliminate the obligatory regulation of cell potassium concentration to maintain the membrane potential. In addition, the extrusion of Na+ and Cl− across the basolateral membrane followed by water would permit the regulation of cell volume and water absorption without significant participation by the Na+/K+ pump. The second sodium pump could also play a similar role in non-epithelial cells, where its contribution to cell volume regulation would be predominant under isotonic conditions.
Finally, it is interesting to note that the expression of the renal and intestinal K+-independent, ouabain-insensitive Na+-ATPase is upregulated by Ang II and is increased in the kidneys of spontaneously hypertensive rats, without modification of the expression of the Na+/K+-ATPase. These observations suggest that the Na+-ATPase, as an essential participant in sodium absorption, could determine the development of salt-dependent essential hypertension. Furthermore, the recognition of particular regulatory sites in its promoter region, different from those identified in the Na+/K+-ATPase gene, opens the possibility that the two enzymes could be differentially regulated under some physiological (e.g., osmotic and volume regulation, hormonal response) or pathophysiological (e.g., essential hypertension, inflammation) conditions.
Future perspectives
The purification and characterization of the Na+-ATPase raises several questions that need to be elucidated. The identification of a putative β-subunit in the purified enzyme, which has not yet been cloned, opens the question whether this subunit is essential for enzyme function or is an insertion chaperone. The answer will probably come from expression experiments. Additionally, the expression of the α or α/β holoenzyme in heterologous systems will allow enough recombinant enzyme to be produced for NMR and crystallization experiments, whereby the functional structure of this protein will be determined. In addition, the recombinant enzyme will permit the exploration of site-directed mutations and thus the identification of essential residues and structural domains. Furthermore, recognition of the inhibitory site for furosemide or triflocin through structural and biochemical studies will allow us to design inhibitory molecules with potential clinical use (e.g. as diuretics). The predictions obtained by in silico analysis will be the starting points for new experimental approaches to elucidate and/or to confirm the biochemical and physiological characteristics of the Na+-ATPase. For instance, the identification of multiple regulatory elements in its promoter region forces detailed molecular analysis of this region and comparison with that of the Na+/K+-ATPase in terms of Na+ transport regulation. The definitive demonstration of the role of Na+-ATPase in pathological states such as inflammatory diseases or essential hypertension will undoubtedly exert a significant impact on medicine.
References
Almers W, Stirling C (1984) Distribution of transport proteins over animal cell membranes. J Membr Biol 77:169–186
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Anckar J, Sistonen L (2007) Heat shock factor 1 as a coordinator of stress and developmental pathways. Adv Exp Med Biol 594:78–88
Andersen JP, Vilsen B (1994) Amino acids Asn796 and Thr799 of the Ca2+-ATPase of sarcoplasmic reticulum bind Ca2+ at different sites. J Biol Chem 269:15931–15936
Armstrong WM, Bixenman WR, Frey KF, Garcia-Diaz JF, O’Regan MG, Owens JL (1979) Energetics of coupled Na+ and Cl− entry into epithelial cells of bullfrog small intestine. Biochim Biophys Acta 551:207–219
Armstrong WM, Byrd BJ, Hamang PM (1973) The Na+ gradient and D galactose accumulation in epithelial cells of bullfrog small intestine. Biochim Biophys Acta 330:237–241
Assaife-Lopes N, Wengert M, de Sá Pinheiro AA, Leão-Ferreira LR, Caruso-Neves C (2009) Inhibition of renal Na+-ATPase activity by inosine is mediated by A1 receptor-induced inhibition of the cAMP signaling pathway. Arch Biochem Biophys 489:76–81
Beltowski J (2010) Leptin and the regulation of renal sodium handling and renal Na+-transporting ATPases: role in the pathogenesis of arterial hypertension. Curr Cardiol Rev 6:31–40
Beltowski J, Borkowska E, Wojcicka G, Marciniak A (2007) Regulation of renal ouabain-resistant Na+-ATPase by leptin, nitric oxide, reactive oxygen species, and cyclic nucleotides: implications for obesity-associated hypertension. Clin Exp Hypertens 29:189–207
Benito B, Garciadeblás B, Rodríguez-Navarro A (2000) Molecular cloning of the calcium and sodium ATPases in Neurospora crassa. Mol Microbiol 35:1079–1088
Bentley PJ (1959) The effects of ionic changes on water transfer across the isolated urinary bladder of the toad Bufo marinus. J Endocrinol 18:327–333
Borgatti AR, Trigari G, Pagliarani A, Ventrella V (1985) Ouabain-insensitive Na+ stimulation of a microsomal Mg2+-ATPase in gills of sea bass (Dicentrarchus labrax L.). Comp Biochem Physiol B Biochem Mol Biol 81:127–135
Breathnach R, Chambon P (1981) Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem 50:349–383
Bröer S (2008) Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev 88:249–286
Bublitz M, Poulsen H, Morth JP, Nissen P (2010) In and out of the cation pumps: P-Type ATPase structure revisited. Curr Opin Struct Biol 20:431–439
Bucher P (1990) Weight matrix descriptions of four eukaryotic RNA polymerase II promoter elements derived from 502 unrelated promoter sequences. J Mol Biol 212:563–578
Cabral LMP, Wengert M, Almeida FG, Caruso-Neves C, Vieyra A, Einicker-Lamas M (2010) Ceramide-activated protein kinases A and C zeta inhibit kidney proximal tubule cell Na+-ATPase. Arch Biochem Biophys 498:57–61
Camejo JL, Proverbio T, Proverbio F (1995) Ouabain-insensitive, Na+-stimulated ATPase activity in rabbit cardiac sarcolemma. Comp Biochem Physiol B Biochem Mol Biol 110:345–348
Candia OA, Zadunaisky J (1972) Potassium flux and sodium transport in the isolated frog skin. Biochim Biophys Acta 255:517–529
Caruccio L, Bae S, Liu AYC, Chen KY (1997) The heat-shock transcription factor HSF1 is rapidly activated by either hyper- or hypo-osmotic stress in mammalian cells. Biochem J 327:341–347
Caruso-Neves C, Einicker-Lamas M, Chagas C, Oliveira MM, Vieyra A, Lopes AG (1999) Ouabain-insensitive Na+-ATPase activity in Trypanosoma cruzi epimastigotes. Zeitschrift fur Naturforschung Section C J Biosci 54:100–104
Caruso-Neves C, Francisco-Pedro LG, Souza LP, Chagas C, Lopes AG (1997) Effect of adenosine on the ouabain-insensitive Na+-ATPase activity from basolateral membrane of the proximal tubule. Biochim Biophys Acta 1329:336–344
Caruso-Neves C, Lara LS, Rangel LBA, Grossi AL, Lopes AG (2000) Angiotensin-(1–7) modulates the ouabain-insensitive Na+-ATPase activity from basolateral membrane of the proximal tubule. Biochim Biophys Acta 1467:189–197
Caruso-Neves C, Lopes AG (2000) Sodium pumps in the malpighian tubule of Rhodnius sp. An Acad Bras Cienc 72:407–412
Caruso-Neves C, Meyer-Fernandes JR, Saad-Nehme J, Proverbio F, Marín R, Lopes AG (1998) Ouabain-insensitive Na+-ATPase activity of Malpighian tubules from Rhodnius prolixus. Comp Biochem Physiol B Biochem Mol Biol 119:807–811
Caruso-Neves C, Provenzano K, Luz FF, Santos FMR, Fernandes MS, Leao-Ferreira LR, Lopes AG (2003) Bradykinin counteracts the stimulatory effect of angiotensin-(1–7) on the proximal tubule Na+-ATPase activity through B2 receptor. Regul Pept 110:207–212
Caruso-Neves C, Siqueira ASE, Iso-Cohen G, Lopes AG (1999) Bradykinin modulates the ouabain-insensitive Na+-ATPase activity from basolateral membrane of the proximal tubule. Biochim Biophys Acta 1431:483–491
Caruso-Neves C, Vives D, Dantas C, Albino CM, Fonseca LM, Lara LS, Iso M, Lopes AG (2004) Ouabain-insensitive Na+-ATPase of proximal tubules is an effector for urodilatin and atrial natriuretic peptide. Biochim Biophys Acta 1660:93–98
Cassano G, Stieger B, Murer H (1984) Na/H− and Cl/OH-exchange in rat jejunal and rat proximal tubular brush border membrane vesicles. Studies with acridine orange. Pflugers Archiv Eur J Physiol 400:309–317
Cho SJ, Huh JE, Song J, Rhee DK, Pyo S (2008) Ikaros negatively regulates inducible nitric oxide synthase expression in macrophages: involvement of Ikaros phosphorylation by casein kinase 2. Cell Mol Life Sci 65:3290–3303
Clarke DM, Loo TW, Inesi G, MacLennan DH (1989) Location of high affinity Ca2+-binding sites within the predicted transmembrane domain of the sarcoplasmic reticulum Ca2+-ATPase. Nature 339:476–478
Cooper ZA, Ghosh A, Gupta A, Maity T, Benjamin IJ, Vogel SN, Hasday JD, Singh IS (2010) Febrile-range temperature modifies cytokine gene expression in LPS-stimulated macrophages by differentially modifying NF-κB recruitment to cytokine gene promoters. Am J Physiol 298:C171–C181
Cornwell RD, Gollahon KA, Hickstein DD (1993) Description of the leukocyte function-associated antigen 1 (LFA-1 or CD11a) promoter. Proc Natl Acad Sci U S A 90:4221–4225
Currant PF, Cereijido M (1965) K fluxes in frog skin. Gen Physiol Biophys 48:1011–1033
Da Silva LL, Cavalcante F, Axelband F, De Souza AM, Lopes AG, Caruso-Neves C (2006) Involvement of the Gi/o/cGMP/PKG pathway in the AT 2-mediated inhibition of outer cortex proximal tubule Na+-ATPase by Ang-(1–7). Biochem J 395:183–190
de Almeida-Amaral EE, Caruso-Neves C, Pires VMP, Meyer-Fernandes JR (2008) Leishmania amazonensis: characterization of an ouabain-insensitive Na+-ATPase activity. Exp Parasitol 118:165–171
De Souza AMD, Batista EJO, Pinheiro AAdS, Carvalhaes M, Lopes AG, De Souza W, Caruso-Neves C (2007) Entamoeba histolytica: ouabain-insensitive Na+-ATPase activity. Exp Parasitol 117:195–200
De Souza AM, Carvalho TLG, Sabino PM, Vives D, Fontes CFL, Lopes AG, Caruso-Neves C (2007) Characterization and partial isolation of ouabain-insensitive Na+-ATPase in MDCK I cells. Biochimie 89:1425–1432
De Souza AM, de Carvalho TLG, LdS L, Gomes-Quintana E, Lopes AG, Caruso-Neves C (2010) The stimulatory effect of angiotensin II on Na+-ATPase activity involves sequential activation of phospholipases and sustained PKC activity. Biochim Biophys Acta 1798:354–359
De Souza AM, Lopes AG, Pizzino CP, Fossari RN, Miguel NCO, Cardozo FP, Abi-Abib R, Fernandes MS, Santos DPA, Caruso-Neves C (2004) Angiotensin II and angiotensin-(1–7) inhibit the inner cortex Na+-ATPase activity through AT2 receptor. Regul Pept 120:167–175
del Castillo JR, Marin R, Proverbio T, Proverbio F (1982) Partial characterization of the ouabain-insensitive, Na+-stimulated ATPase activity of kidney basal-lateral plasma membranes. Biochim Biophys Acta 692:61–68
del Castillo JR, Robinson JWL (1982) The simultaneous preparation of basolateral and brush-border membrane-vesicles from guinea-pig intestinal epithelium, and the determination of the orientation of the basolateral vesicles. Biochim Biophys Acta 688:45–56
del Castillo JR, Robinson JWL (1985) Mg2+-ATP-dependent sodium-transport in inside-out basolateral plasma-membrane vesicles from guinea-pig small intestinal epithelial-cells. Biochim Biophys Acta 812:402–412
del Castillo JR, Robinson JWL (1985) Na+-stimulated ATPase activities in basolateral plasma membranes from guinea pig small intestinal epithelial cells. Biochim Biophys Acta 812:413–422
del Castillo JR, Romero FJ, Thomas LE, Cariani L (2005) Phosphorylated intermediate of the Na-ATPase associated with second sodium pump. FEBS J 272:188–188
del Castillo JR, Whittembury G (1987) Na+, K+ and Cl- transport in isolated small intestinal cells from guinea pig. Evidences for the existence of a second Na+ pump. Biochim Biophys Acta 901:209–216
Di Campo V, Hendriquez LM, Proverbio T, Marín R, Proverbio F (1991) Na+-ATPase activity, cell ion and water contents of kidney cortex slices from rats on a high Na+ diet. Biomed Biochim Acta 50:1213–1216
Diamond JM, Bossert WH (1968) Functional consequences of ultrastructural geometry in “backwards” fluid-transporting epithelia. J Cell Biol 37:694–702
DiBona DR, Mills JW (1979) Distribution of Na+-pump sites in transporting epithelia. Fed Proc 38:134–143
Diecke FP, Cacace VI, Montalbetti N, Ma L, Kuang K, Iserovich P, Fischbarg J (2011) Comparative permeabilities of the paracellular and transcellular pathways of corneal endothelial layers. J Membr Biol 242:41–51
Drozdowski L, Thomson ABR (2006) Intestinal sugar transport. World J Gastroenterol 12:1657–1670
Ernst SA, Hootman SR (1981) Microscopical methods for the localization of Na+, K+-ATPase. Histochem J 13:397–418
Essig A (1965) Active sodium transport in toad bladder despite removal of serosal potassium. Am J Physiol 208:401–406
Essig A, Frazier HS, Leaf A (1963) Evidence for ‘electrogenic’ active sodium transport in an epithelial membrane. Nature 197:701
Eveloff JR, Kinne R, Kinne-Saffran EM, Murer H, Silva P, Epstein H, Stoff J, Kinter WB (1978) Coupled sodium and chloride transport into plasma membrane vesicles prepared from dogfish rectal gland. Pflugers Archiv Eur J Physio 378:87–92
Fordtran JS, Ingelfinger FJ (1968) Absorption of water, electrolytes and sugars from human gut. In: Code CF, Heidel W (eds) Handbook of physiology, section 6, alimentary canal III. Intestinal absorption. Williams and Wilkins, Baltimore, pp 1457–1490
Fordtran JS, Locklear TW (1966) Ionic constituents and osmolality of gastric and small-intestinal fluids after eating. Am J Dig Dis 11:503–521
Gamba G (2009) The thiazide-sensitive Na_-Cl_ cotransporter: molecular biology, functional properties, and regulation by WNKs. Am J Physiol 297:F838–F848
Ganapathy VGN, Martindale RG (2006) Protein digestion and absorption. In: Johnson L, Barret K, Gishan F, Merchant J, Said H, Wood J (eds) Physiology of gastrointestinal tract, 4th edn. Academic, Baltimore, pp 1667–1692
Garty H, Palmer LG (1997) Epithelial sodium channels: function, structure and regulation. Physiol Rev 77:359–396
Gerencser GA, Zhang J (2003) Chloride ATPase pumps in nature: do they exist? Biol Rev Camb Philos Soc 78:197–218
Giebisch G, Sullivan LP, Whittembury G (1973) Relationship between tubular net sodium reabsorption and peritubular potassium uptake in the perfused Necturus kidney. J Physiol 230:51–74
Gomes CP, Leao-Ferreira LR, Caruso-Neves C, Lopes AG (2005) Adenosine reverses the stimulatory effect of angiotensin II on the renal Na+-ATPase activity through the A2 receptor. Regul Pept 129:9–15
Gomes CP, Leao-Ferreira LR, Pinheiro AAS, Gomes-Quintana E, Wengert M, Lopes AG, Caruso-Neves C (2008) Crosstalk between the signaling pathways triggered by angiotensin II and adenosine in the renal proximal tubules: implications for modulation of Na+-ATPase activity. Peptides 29:2033–2038
Gorvel JP, Liabeuf A, Massey D, Liot D, Goridis C, Maroux S (1983) Recognition of sodium- and potassium-dependent adenosine triphosphatase in organs of the mouse by means of a monoclonal antibody. Cell Tissue Res 234:619–632
Hediger MA, Coady MJ, Ikeda TS, Wright EM (1987) Expression cloning and cDNA sequencing of the Na+/glucose co-transporter. Nature 330:379–381
Heinemeyer T, Wingender E, Reuter I, Hermjakob H, Kel AE, Kel OV, Ignatieva EV, Ananko EA, Podkolodnaya OA, Kolpakov FA, Podkolodny NL, Kolchanov NA (1998) Databases on transcriptional regulation: TRANSFAC, TRRD and COMPEL. Nucleic Acids Res 26:362–367
Heitzmann D, Warth R (2008) Physiology and pathophysiology of potassium channels in gastrointestinal epithelia. Physiol Rev 88:1119–1182
Hilge M, Siegal G, Vuister GW, Güntert P, Gloor SM, Abrahams JP (2003) ATP-induced conformational changes of the nucleotide-binding domain of Na, K-ATPase. Nat Struct Biol 10:468–474
Imagawa T, Yamamoto T, Kaya S, Sakaguchi K, Taniguchi K (2005) Thr-774 (transmembrane segment M5), Val-920 (M8), and Glu-954 (M9) are involved in Na+ transport, and Gln-923 (M8) is essential for Na, K-ATPase activity. J Biol Chem 280:18736–18744
John LB, Ward AC (2011) The Ikaros gene family: transcriptional regulators of hematopoiesis and immunity. Mol Immunol 48:1272–1278
Jorge F (2010) Fluid transport across leaky epithelia: central role of the tight junction and supporting role of aquaporins. Physiol Rev 90:1271–1290
Kadem O, Katchalsky A (1958) Thermodynamic analysis of the permeability of biologicalmembranes to non-electrolytes. Biochim Biophys Acta 27:229–246
Kiela PR, Xu H, Ghishan FK (2006) Apical Na+/H+ exchangers in the mammalian gastrointestinal tract. J Physiol Pharmacol 57(suppl 7):51–79
Kinne R, Kinne-Saffran EM (1978) Differentiation of cell faces in epithelia. In: Solomon AK, Karnovsky M (eds) Molecular specialization and symmetry in membrane function. Harvard University Press, Cambridge, pp 272–293
Kühlbrandt W (2004) Biology, structure and mechanism of P-type ATPases. Nat Rev Mol Cell Biol 5:282–295
Lagerspetz KYH, Pivovarova NB, Senius KEO (1992) Monovalent cation activated ouabain-insensitive ATPase in the gills of freshwater mussel Anodonta cygnea. Comp Biochem Physiol B Biochem Mol Biol 103:903–908
Lara LS, Bica RBS, Sena SLF, Correa JS, Marques-Fernandes MF, Lopes AG, Caruso-Neves C (2002) Angiotensin-(1–7) reverts the stimulatory effect of angiotensin II on the proximal tubule Na+-ATPase activity via a A779-sensitve receptor. Regul Pept 103:17–22
Lara LS, Correa JS, Lavelle AB, Lopes AG, Caruso-Neves C (2008) The angiotensin receptor type 1-Gq protein-phosphatidyl inositol phospholipase Cβ-protein kinase C pathway is involved in activation of proximal tubule Na+-ATPase activity by angiotensin(1–7) in pig kidneys. Exp Physiol 93:639–647
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948
Lee CO, Armstrong WM (1972) Activities of sodium and potassium ions in epithelial cells of small intestine. Science 175:1261–1264
Levitt DG, Hakim AA, Lifson N (1969) Evaluation of components of transport of sugars by dog jejunum in vivo. Am J Physiol 217:777–783
Líbano-Soares JD, Landgraf SS, Gomes-Quintana E, Lopes AG, Caruso-Neves C (2011) Prostaglandin E2 modulates proximal tubule Na+-ATPase activity: cooperative effect between protein kinase A and protein kinase C. Arch Biochem Biophys 507:281–286
Liedtke CM, Hopfer U (1977) Anion transport in brush border membranes isolated from rat small intestine. Biochem Biophys Res Commun 76:579–585
Lifson N, Gruman LM, Levitt DG (1968) Diffusive-convective models for intestinal absorption of D2O. Am J Physiol 215:444–454
Lifson N, Hakim AA (1966) Simple diffusive-convective model for intestinal absorption of a nonelectrolyte (urea). Am J Physiol 211:1137–1146
Lizumi K, Mikami Y, Hashimoto M, Nara T, Hara Y, Aoki T (2006) Molecular cloning and characterization of ouabain-insensitive Na+-ATPase in the parasitic protist, Trypanosoma cruzi. Biochim Biophys Acta 1758:738–746
Loo DDF, Wright EM, Zeuthen T (2002) Water pumps. J Physiol 542:53–60
Lopes AG, Soares AC, Santos DPA, Fernandes MS, Leão-Ferreira LR, Quintana-Gomes E, Caruso-Neves C (2004) PLA2/PGE2 are involved in the inhibitory effect of bradykinin on the angiotensin-(1–7)-stimulated Na+-ATPase activity of the proximal tubule. Regul Pept 117:37–41
Lutsenko S, Kaplan JH (1995) Organization of P-type ATPases: significance of structural diversity. Biochemistry 34:15607–15613
Macknight ADC, Civan MM, Leaf A (1975) The sodium transport pool in toad urinary bladder epithelial cells. J Membr Biol 20:365–386
Macknight ADC, Civan MM, Leaf A (1975) Some effects of ouabain on cellular ions and water in epithelial cells of toad urinary bladder. J Membr Biol 20:387–401
Mailman DS (1981) Fluid and electrolyte absorption. In: Johnson LR (ed) Gastrointestinal physiology, 2nd edn. Mosby, St. Louis, pp 107–122
Malakooti J, Saksena S, Gill RK, Dudeja PK (2011) Transcriptional regulation of the intestinal luminal Na+ and Cl− transporters. Biochem J 435:313–325
Marin R, Obando MA, Proverbio T, Proverbio F (1986) Effect of a high NaCl diet on the active mechanisms of Na+ extrusion in rat kidney. Kidney Int 30:518–523
Marin R, Proverbio T, Proverbio F (1985) Active sodium transport in basolateral plasma membrane vesicles from rat kidney proximal tubular cells. Biochim Biophys Acta 814:363–373
Marin R, Proverbio T, Proverbio F (1985) Characterization of the Na+, K+-ATPase activity of basolateral plasma membranes of kidney proximal tubular cells from young and old rats. Biochem Pharmacol 34:4197–4201
Marín R, Proverbio T, Proverbio F (1985) Effect of Ca2+ on the ouabain-insensitive, active Na+ uptake in inside–out basolateral plasma membrane vesicles from rat kidney proximal tubular cells. Biochim Biophys Acta 817:299–306
Marín R, Rodriguez AJ, Proverbio T (1992) Partial characterization of the inhibitory effect of lipid peroxidation on the ouabain-insensitive Na-ATPase of rat kidney cortex plasma membranes. J Bioenerg Biomembr 24:329–335
Marxer A, Stieger B, Quaroni A, Kashgarian M, Hauri HP (1989) (Na+ + K+)-ATPase and plasma membrane polarity of intestinal epithelial cells: presence of a brush border antigen in the distal large intestine that is immunologically related to β subunit. J Cell Biol 109:1057–1069
Møller JV, Juul B, Le Maire M (1996) Structural organization, ion transport, and energy transduction of P-type ATPases. Biochim Biophys Acta 1286:1–51
Moretti R, Martin M, Proverbio T, Proverbio F, Marin R (1991) Ouabain-insensitive Na-ATPase activity in homogenates from different animal tissues. Comp Biochem Physiol B Biochem Mol Biol 98:623–626
Morth JP, Pedersen BP, Toustrup-Jensen MS, Sørensen TLM, Petersen J, Andersen JP, Vilsen B, Nissen P (2007) Crystal structure of the sodium–potassium pump. Nature 450:1043–1049
Murer H, Hopfer U (1974) Demonstration of electrogenic Na+ dependent D glucose transport in intestinal brush border membranes. Proc Natl Acad Sci U S A 71:484–488
Murer H, Hopfer U, Kinne R (1976) Sodium/proton antiport in brush border membrane vesicles isolated from rat small intestine and kidney. Biochem J 154:597–604
Murer H, Hopfer U, Kinne Saffran E, Kinne R (1974) Glucose transport in isolated brush border and lateral basal plasma membrane vesicles from intestinal epithelial cells. Biochim Biophys Acta 345:170–179
Nagarsekar A, Hasday JD, Singh IS (2005) CXC chemokines: a new family of heat-shock proteins? Immunol Investig 34:381–398
Nellans HN, Schultz SG (1976) Relations among transepithelial sodium transport, potassium exchange, and cell volume in rabbit ileum. J Gen Physiol 68:441–463
Nielsen M, Lundegaard C, Lund O, Petersen TN (2010) CPHmodels-3.0—remote homology modeling using structure-guided sequence profiles. Nucleic Acids Res 38:W576–W581
O’Doherty J, Garcia-Diaz JF, Armstrong McD W (1979) Sodium-selective liquid ion-exchanger microelectrodes for intracellular measurements. Science 203:1349–1351
Obando MA, Marin R, Proverbio T, Proverbio F (1987) High sodium diet and Na+-stimulated ATPase activities in basolateral plasma membranes from rat kidney proximal tubular cells. Biochem Pharmacol 36:7–11
Ogawa H, Shinoda T, Cornelius F, Toyoshima C (2009) Crystal structure of the sodium–potassium pump (Na+, K+-ATPase) with bound potassium and ouabain. Proc Natl Acad Sci U S A 106:13742–13747
Pagliarani A, Ventrella V, Trombetti F, Trigari G, Borgatti AR (1988) (Na+ + K+)- and Na+-stimulated Mg2+-dependent ATPase activities in kidney of sea bass (Dicentrarchus labrax L.). Comp Biochem Physiol B Biochem Mol Biol 90:41–52
Palmgren MG, Axelsen KB (1998) Evolution of P-type ATPases. Biochim Biophys Acta 1365:37–45
Preiss R, Busse E, Banaschak H (1979) An ouabain-insensitive Na-ATPase of the arterial vascular muscle cell and its relation to ouabain-sensitive Na, K-ATPase. Acta Biologica et Medica Germanica 38:1387–1397
Proverbio F, Condrescu Guidi M, Whittembury G (1975) Ouabain insensitive Na+ stimulation of an Mg2+ dependent ATPase in kidney tissue. Biochim Biophys Acta 394:281–292
Proverbio F, del Castillo JR (1981) Na+-stimulated ATPase activities in kidney basal-lateral plasma membranes. Biochim Biophys Acta 646:99–108
Proverbio F, Duque JA, Proverbio T, Marin R (1988) Cell volume-sensitive Na+-ATPase activity in rat kidney cortex cell membranes. Biochim Biophys Acta 941:107–110
Proverbio T, Marín R, Proverbio F (1988) Ouabain-insensitive, Na+-stimulated ATPase activity in squid gill microsomes. Comp Biochem Physiol 90:341–345
Proverbio F, Marin R, Proverbio T (1991) The ouabain-insensitive sodium pump. Comp Biochem Physiol Physiol 99:279–283
Proverbio F, Proverbio T, Marin R (1982) Ouabain-insensitive Na+-stimulated ATPase activity of basolateral plasma membranes from guinea-pig kidney cortex cells. II. Effect of Ca2+. Biochim Biophys Acta 688:757–763
Proverbio F, Proverbio T, Marin R (1985) Ion transport and oxygen consumption in kidney cortex slices from young and old rats. Gerontology 31:166–173
Proverbio F, Proverbio T, Matteo RG, Perrone TM, Marin R (1988) Na-pump activity in rat kidney cortex cells and its relationship with the cell volume. FEBS Lett 236:318–320
Proverbio F, Robinson JWL, Whittembury G (1970) Sensitivities of (Na+K+)-ATPase and Na+ extrusion mechanisms to ouabain and ethacrynic acid in the cortex of the guinea-pig kidney. Biochim Biophys Acta 211:327–336
Proverbio F, Whittembury G (1975) Cell electrical potentials during enhanced sodium extrusion in guinea pig kidney cortex slices. J Physiol 250:559–578
Proverbio F, Yaris GI, Proverbio T, Marín R (1986) Effect of norepinephrine on NaCl extrusion in guinea-pig kidney cortex slices. Acta Cient Venez 37:519–525
Proverbio T, Zanders IP, Marin R, Rodriguez JM, Proverbio F (1990) Effects of Na+ and/or K+ on the Mg2+-dependent ATPase activities in shrimp (Macrobrachium amazonicum) gill homogenates. Comp Biochem Physiol B Biochem Mol Biol 97:383–390
Queiroz-Madeira EP, Lara LS, Wengert M, Landgraf SS, Líbano-Soares JD, Zapata-Sudo G, Sudo RT, Takiya CM, Gomes-Quintana E, Lopes AG, Caruso-Neves C (2010) Na+-ATPase in spontaneous hypertensive rats: possible AT1 receptor target in the development of hypertension. Biochim Biophys Acta 1798:360–366
Quigley JP, Gotterer GS (1972) A comparison of the (Na+-K+)-ATPase activities found in isolated brush border and plasma membrane of the rat intestinal mucosa. Biochim Biophys Acta 255:107–113
Rangel LBA, Caruso-Neves C, Lara LS, Brasil FL, Lopes AG (1999) Angiotensin II activates the ouabain-insensitive Na+-ATPase from renal proximal tubules through a G-protein. Biochim Biophys Acta 1416:309–319
Rangel LBA, Caruso-Neves C, Lara LS, Lopes AG (2002) Angiotensin II stimulates renal proximal tubule Na+-ATPase activity through the activation of protein kinase C. Biochim Biophys Acta 1564:310–316
Rangel LBA, Lopes AG, Lara LSM, Carvalho TLG, Silva IV, Oliveira MM, Einicker-Lamas M, Vieyra A, Nogaroli L, Caruso-Neves C (2005) PI-PLCβ is involved in the modulation of the proximal tubule Na+-ATPase by angiotensin II. Regul Pept 127:177–182
Rangel LBA, Malaquias AT, Lara LS, Silva IV, De Souza AM, Lopes AG, Caruso-Neves C (2001) Protein kinase C-induced phosphorylation modulates the Na+-ATPase activity from proximal tubules. Biochim Biophys Acta 1512:90–97
Reese MG (2001) Application of a time-delay neural network to promoter annotation in the Drosophila melanogaster genome. Comput Chem 26:51–56
Renfro JL (1977) Interdependence of active Na+ and Cl− transport by the isolated urinary bladder of the teleost, Pseudopleuronectes americanus. J Exp Zool 199:383–390
Reyes A, Galindo MM, García L, Segura-Peña D, Caruso-Neves C, Eblen-Zajjur A, Proverbio T, Marín R, Proverbio F (2009) Ouabain-insensitive, Na+-stimulated ATPase of several rat tissues: activity during a 24 h period. Physiol Res 58:693–699
Robinson BA, Macknight ADC (1976) Relationships between serosal medium potassium concentration and sodium transport in toad urinary bladder. I. Effects of different medium potassium concentrations on electrical parameters. J Membr Biol 26:217–238
Robinson BA, Macknight ADC (1976) Relationships between serosal medium potassium concentration and sodium transport in toad urinary bladder. II. Effects of different medium potassium concentrations on epithelial cell composition. J Membr Biol 26:239–268
Robinson BA, Macknight ADC (1976) Relationships between serosal medium potassium concentration and sodium transport in toad urinary bladder. III. Exchangeability of epithelial cellular potassium. J Membr Biol 26:269–286
Rocafull MA, Romero FJ, Thomas LE, Del Castillo JR (2011) Isolation and cloning of the K+-independent, ouabain-insensitive Na+-ATPase. Biochim Biophys Acta 1808:1684–1700
Rocafull MA, Thomas LE, Barrera GJ, del Castillo JR (2010) Differential expression of P-type ATPases in intestinal epithelial cells: identification of putative new atp1a1 splice-variant. Biochem Biophys Res Commun 391:152–158
Rossier BC, Pradervand S, Schild L, Hummler E (2002) Epithelial sodium channel and the control of sodium balance: interaction between genetic and environmental factors. Annu Rev Physiol 64:877–897
Rothman A, Proverbio T, Proverbio F (1996) Inhibitory effect of ethanol on the Na+-ATPase activity of rat kidney proximal tubular cell plasma membranes. Physiol Res 45:205–211
Sacktor B (1977) Transport in membrane vesicles isolated from the mammalian kidney and intestine. Curr Top Bioenerg 6:39–81
Saraiva VB, Wengert M, Gomes-Quintana E, Heise N, Caruso-Neves C (2009) Na+-ATPase and protein kinase C are targets to 1-O-hexadecylphosphocoline (miltefosine) in Trypanosoma cruzi. Arch Biochem Biophys 481:65–71
Schild L (2010) The epithelial sodium channel and the control of sodium balance. Biochim Biophys Acta 1802:1159–1165
Schultz SG (1977) The role of paracellular pathways in isotonic fluid transport. Yale J Biol Med 50:99–113
Schultz SG (1977) Sodium-coupled solute transport of small intestine: a status report. Am J Physiol 233:E249–E254
Schultz SG (1978) Ion-coupled transport across biological membranes. In: Andreoli TE, Hoffman JF, Fanestil DD (eds) Physiology of membrane disorders. Plenum, New York, pp 273–286
Schultz SG (1979) Transport across small intestine. In: Giebisch G, Tostenson DC, Using HH (eds) Membrane transport in biology, volume IV-B IV B. Springer, Heidelberg–New York, pp 749–780
Schultz SG, Curran PF (1970) Coupled transport of sodium and organic solutes. Physiol Rev 50:637–718
Schultz SG, Curran PF (1974) Sodium and chloride transport across isolated rabbit IIeum. Curr Top Membr Transport 5:225–281
Schultz SG, Frizzell RA (1972) An overview of intestinal absorptive and secretory processes. Gastroenterology 63:161–170
Schultz SG, Frizzell RA, Nellans HN (1974) Ion transport by mammalian small intestine. Annu Rev Physiol 36:51–91
Sepulveda FV, Burton KA, Brown PD (1982) Relation between sodium-coupled amino acid and sugar transport and sodium/potassium pump activity in isolated intestinal epithelial cells. J Cell Physiol 111:303–308
Shachar-Hill B, Hill AE (2002) Paracellular fluid transport by epithelia. Int Rev Cytol 215:319–350
Shinoda T, Ogawa H, Cornelius F, Toyoshima C (2009) Crystal structure of the sodium-potassium pump at 2.4 resolution. Nature 459:446–450
Shono M, Wada M, Hara Y, Fujii T (2001) Molecular cloning of Na+-ATPase cDNA from a marine alga, Heterosigma akashiwo. Biochim Biophys Acta 1511:193–199
Smale ST, Baltimore D (1989) The ‘initiator’ as a transcription control element. Cell 57:103–113
Snider C, Jayasinghe S, Hristova K, White SH (2009) MPEx: a tool for exploring membrane proteins. Protein Sci 18:2624–2628
Stirling CE (1972) Radioautographic localization of sodium pump sites in rabbit intestine. J Cell Biol 53:704–714
Tanaka KI, Mizushima T (2009) Protective role of HSF1 and HSP70 against gastrointestinal diseases. Int J Hyperth 25:668–676
Thiagarajah J, Verkman A (2006) Water transport in the gastrointestinal tract. In: Johnson L, Barret K, Gishan F, Merchant J, Said H, Wood J (eds) Physiology of the gastrointestinal tract, 4th edn. Academic, Baltimore, pp 1827–1845
Thomas LE, Burguillos L, del Castillo JR (2003) Backdoor phosphorylation of basolateral plasma membranes of small intestinal epithelial cells: characterization of a furosemide-induced phosphoprotein related to the second sodium pump. Arch Biochem Biophys 419:190–197
Tirri R, Tuomola P, Bowler K (1980) The presence of a Na+ATPase activity associated with mammalian brain microsomal fractions. Int J Biochem 11:43–48
Tosco M, Orsenigo MN, Esposito G, Faelli A (1988) Ouabain-insensitive active sodium transport in rat jejunum: evidence from ATPase activities, Na uptake by basolateral membrane vesicles and in vitro transintestinal transport. Cell Biochem Funct 6:155–164
Tosco M, Orsenigo MN, Esposito G, Faelli A (1988) Ouabain-insensitive transintestinal transport in the rat jejunum incubated in vitro. Proc Soc Exp Biol Med 188:122–127
Tosco M, Orsenigo MN, Zoppi S, Faelli A (1990) Aging and ATPase activities in rat jejunum. Mech Ageing Dev 56:265–274
Toyoshima C, Nakasako M, Nomura H, Ogawa H (2000) Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 Å resolution. Nature 405:647–655
Turnberg LA, Bieberdorf FA, Morawski SG, Fordtran JS (1970) Interrelationships of chloride, bicarbonate, sodium, and hydrogen transport in the human ileum. J Clin Investig 49:557–567
Umetsu SE, Winandy S (2009) Ikaros is a regulator of Il10 expression in CD4+ T cells. J Immunol 183:5518–5525
Ventrella V, Elvir JR, Borgatti AR, Trigari G, Proverbio T, Pagliarani A, Trombetti F, Pirini M, Marín R, Proverbio F (2010) Phosphorylated intermediate of the ouabain-insensitive, Na+-stimulated ATPase in rat kidney cortex and rainbow trout gills. Biochimie 92:128–135
Ventrella V, Trombetti F, Pagliarani A, Trigari G, Borgatti AR (1990) Gill (Na+ + K+)- and Na+-stimulated Mg2+-dependent ATPase activities in the gilthead bream (Sparus auratus L.). Comp Biochem Physiol B Biochem Mol Biol 95:95–105
Ventrella V, Trombetti F, Pagliarani A, Trigari G, Pirini M, Borgatti AR (1992) Salinity dependence of the ouabain-insensitive Mg2+-dependent Na+-ATPase in gills of rainbow trout (Oncorhynchus mykiss Walbaum) adapted to fresh and brackish water. Comp Biochem Physiol B Biochem Mol Biol 101:1–7
Visscher MB, Varco RH, Carr CW, Dean RB, Erickson D (1944) Sodium ion movement between the intestinal lumen and the blood. Am J Physiol 141:488–505
Vives D, Farage S, Motta R, Lopes AG, Caruso-Neves C (2010) Atrial natriuretic peptides and urodilatin modulate proximal tubule Na+-ATPase activity through activation of the NPR-A/cGMP/PKG pathway. Peptides 31:903–908
Wengert M, Adao-Novaes J, Assaife-Lopes N, Leao-Ferreira LR, Caruso-Neves C (2007) Adenine-induced inhibition of Na+-ATPase activity: evidence for involvement of the Gi protein-coupled receptor in the cAMP signaling pathway. Arch Biochem Biophys 467:261–267
Wengert M, Adao-Novaes J, Leao-Ferreira LR, Caruso-Neves C (2011) Guanine-induced inhibition of renal Na+-ATPase activity: evidence for the involvement of the Gi protein-coupled receptor. Arch Biochem Biophys 513:126–130
Wengert M, Berto C Jr, Kaufman J, Leao-Ferreira LR, Paes-De-Carvalho R, Lopes AG, Caruso-Neves C (2005) Stimulation of the proximal tubule Na+-ATPase activity by adenosine A2A receptor. Int J Biochem Cell Biol 37:155–165
Whittembury G, Proverbio F (1970) Two modes of Na extrusion in cells from guinea pig kidney cortex slices. Pflugers Archiv Eur J Physio 316:1–25
Wright EM (2001) Renal Na+-glucose cotransporters. Am J Physiol 280:F10–F18
Wright E, Loo D, Hirayama B, Turk E (2006) Sugar absorption. In: Johnson L, Barret K, Gishan F, Merchant J, Said H, Wood J (eds) Physiology of gastrointestinal tract, 4th edn. Academic, Baltimore, pp 1653–1665
Wright EM, Van Os CH, Mircheff AK (1980) Sugar uptake by intestinal basolateral membrane vesicles. Biochim Biophys Acta 597:112–124
Yaris GI, Marín R, Proverbio T, Proverbio F (1988) Adrenergic receptors and active ion transport of kidney cortex slices in guinea pigs. Acta Cient Venez 39:237–244
Zeuthen T (2002) General models for water transport across leaky epithelia. Int Rev Cytol 215:285–317
Acknowledgments
We thank Mr. Victor Salazar for immunohistochemical technical assistance. This work was partly supported by “Misión Ciencia” Grant 20070015856. English editing service was performed by BioMedES, The University of Aberdeen, Scotland, UK.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Rocafull, M.A., Thomas, L.E. & del Castillo, J.R. The second sodium pump: from the function to the gene. Pflugers Arch - Eur J Physiol 463, 755–777 (2012). https://doi.org/10.1007/s00424-012-1101-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-012-1101-3