Abstract
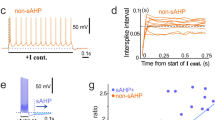
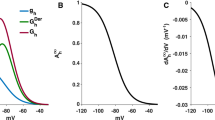
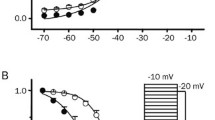
Whole-cell, patch-clamp recordings were carried out in acutely dissociated neurons from entorhinal cortex (EC) layer II to study the effects of Zn2+ on Na+ current kinetics and voltage dependence. In the presence of 200 μM extracellular Cd2+ to abolish voltage-dependent Ca2+ currents, and 100 mM extracellular Na+, 1 mM Zn2+ inhibited the transient Na+ current, I NaT, only to a modest degree (~17% on average). A more pronounced inhibition (~36%) was induced by Zn2+ when extracellular Na+ was lowered to 40 mM. Zn2+ also proved to modify I NaT voltage-dependent and kinetic properties in multiple ways. Zn2+ (1 mM) shifted the voltage dependence of I NaT activation and that of I NaT onset speed in the positive direction by ~5 mV. The voltage dependence of I NaT steady-state inactivation and that of I NaT inactivation kinetics were markedly less affected by Zn2+. By contrast, I NaT deactivation speed was prominently accelerated, and its voltage dependence was shifted by a significantly greater amount (~8 mV on average) than that of I NaT activation. In addition, the kinetics of I NaT recovery from inactivation were significantly slowed by Zn2+. Zn2+ inhibition of I NaT showed no signs of voltage dependence over the explored membrane-voltage window, indicating that the above effects cannot be explained by voltage dependence of Zn2+-induced channel-pore block. These findings suggest that the multiple, voltage-dependent state transitions that the Na+ channel undergoes through its activation path are differentially sensitive to the gating-modifying effects of Zn2+, thus resulting in differential modifications of the macroscopic current’s activation, inactivation, and deactivation. Computer modeling provided support to this hypothesis.










Similar content being viewed by others
References
Aniksztejn L, Charton G, Ben-Ari Y (1987) Selective release of endogenous zinc from the hippocampal mossy fibers in situ. Brain Res 404:58–64
Assaf SY, Chung SH (1984) Release of endogenous Zn2+ from brain tissue during activity. Nature 308:73473–73476
Balser JR (1999) Structure and function of the cardiac sodium channels. Cardiovasc Res 42:327–338
Bardoni R, Belluzzi O (1994) Modifications of A-current kinetics in mammalian central neurones induced by extracellular zinc. J Physiol 479:389–400
Castelli L, Nigro MJ, Magistretti J (2007) Analysis of resurgent sodium-current expression in rat parahippocampal cortices and hippocampal formation. Brain Res 1163:44–55
Castelli L, Tanzi F, Taglietti V, Magistretti J (2003) Cu2+, Co2+, and Mn2+ modify the gating kinetics of high-voltage-activated Ca2+ channels in rat palaeocortical neurons. J Membr Biol 195:121–136
Constanti A, Smart TG (1987) Zinc blocks the A-current in cultured rat sympathetic neurones. J Physiol 396:159
Cuajungco MP, Lees GJ (1997) Zinc metabolism in the brain: relevance to human neurodegenerative disorders. Neurobiol Dis 4:137–169
Davidson JL, Kehl SJ (1995) Changes of activation and inactivation gating of the transient potassium current of rat pituitary melanotrophs caused by micromolar Cd2+ and Zn2+. Can J Physiol Pharmacol 73:36–42
Fozzard HA, Hanck DA (1996) Structure and function of voltage-dependent sodium channels: comparison of brain II and cardiac isoforms. Physiol Rev 76:887–926
Frederickson CJ (1989) Neurobiology of zinc and zinc-containing neurons. Int Rev Neurobiol 31:145–238
Hanck DA, Sheets MF (1992) Extracellular divalent and trivalent cation effects on sodium current kinetics in single canine cardiac Purkinje cells. J Physiol 454:267–298
Hille B (2001) Ion channels of excitable membranes. Sinauer, Sunderland
Hines ML, Carnevale NT (1997) The NEURON simulation environment. Neural Comput 9:1179–1209
Horn R, Vandenberg CA (1984) Statistical properties of single sodium channels. J Gen Physiol 84:505–534
Horn R, Vandenberg CA, Lange K (1984) Statistical analysis of single sodium channels. Effects of N-bromoacetamide. Biophys J 45:323–335
Howell GA, Welch MG, Frederickson CJ (1984) Stimulation-induced uptake and release of zinc in hippocampal slices. Nature 308:736–738
Irvine LA, Jafri MS, Winslow RL (1999) Cardiac sodium channel Markov model with temperature dependence and recovery from inactivation. Biophys J 76:1868–1885
Kerchner GA, Canzoniero LM, Yu SP, Ling C, Choi DW (2000) Zn2+ current is mediated by voltage-gated Ca2+ channels and enhanced by extracellular acidity in mouse cortical neurones. J Physiol 528:39–52
Kuo CC, Bean BP (1994) Na+ channels must deactivate to recover from inactivation. Neuron 12:819–829
Magistretti J, Alonso A (1999) Biophysical properties and slow voltage-dependent inactivation of a sustained sodium current in entorhinal cortex layer-II principal neurons: a whole-cell and single-channel study. J Gen Physiol 114:491–509
Magistretti J, Brevi S, de Curtis M (2001) Ni2+ slows the activation kinetics of high-voltage-activated Ca2+ currents in cortical neurons: evidence for a mechanism of action independent of channel-pore block. J Membr Biol 179:243–262
Magistretti J, Castelli L, Taglietti V, Tanzi F (2003) Dual effect of Zn2+ on multiple types of voltage-dependent Ca2+ currents in rat palaeocortical neurons. Neuroscience 117:249–264
Mathie A, Sutton GL, Clarke CE, Veale EL (2006) Zinc and copper: pharmacological probes and endogenous modulators of neuronal excitability. Pharmacol Ther 111:567–583
Mayer ML, Vyklicky L Jr (1989) The action of zinc on synaptic transmission and neuronal excitability in cultures of mouse hippocampus. J Physiol 415:351–365
Peters S, Koh J, Choi DW (1987) Zinc selectively blocks the action of N-methyl-d-aspartate on cortical neurons. Science 236:589–593
Satin J, Kyle JW, Chen M, Rogart RB, Fozzard HA (1992) The cloned cardiac Na channel α-subunit expressed in Xenopus oocytes shows gating and blocking properties of native channels. J Membr Biol 130:11–22
Sheets MF, Hanck DA (1995) Voltage-dependent open-state inactivation of cardiac sodium channels: gating current studies with Anthopleurin-A toxin. J Gen Physiol 106:617–640
Smart TG, Constanti A (1990) Differential effect of zinc on the vertebrate GABAA-receptor complex. Br J Pharmacol 99:643–654
Smart TG, Xie X, Krishek BJ (1994) Modulation of inhibitory and excitatory amino acid receptor ion channels by zinc. Prog Neurobiol 42:393–341
Smith MR, Smith RD, Plummer NW, Meisler MH, Goldin AL (1998) Functional analysis of the mouse Scn8a sodium channel. J Neurosci 18:6093–6102
Spires S, Begenisich T (1992) Chemical properties of the divalent cation binding site on potassium channels. J Gen Physiol 100:181–193
Taddese A, Bean BP (2002) Subthreshold sodium current from rapidly inactivating sodium channels drives spontaneous firing of tuberomammillary neurons. Neuron 33:587–600
Vandenberg CA, Bezanilla F (1991) A sodium channel gating model based on single channel, macroscopic ionic, and gating currents in the squid giant axon. Biophys J 60:1511–1533
Visentin S, Zaza A, Ferroni A, Tromba C, DiFrancesco C (1990) Sodium current block caused by group IIb cations in calf Purkinje fibres and in guinea-pig ventricular myocytes. Pflügers Arch 417:213–222
Weiss JH, Sensi SL, Koh JY (2000) Zn2+: a novel ionic mediator of neural injury in brain disease. Trends Pharmacol Sci 21:395–401
Westbrook GL, Mayer ML (1987) Micromolar concentrations of Zn2+ antagonize NMDA and GABA responses of hippocampal neurons. Nature 328:640–643
White JA, Alonso A, Kay AR (1993) A heart-like Na+ current in the medial entorhinal cortex. Neuron 11:1037–1047
Zeng D, Kyle JW, Martin RL, Ambler KS, Hanck DA (1996) Cardiac sodium channels expressed in a peripheral neurotumor-derived cell line, RT4-B8. Am J Physiol 270:C1522–1531
Zhang S, Kehl SJ, Fedida D (2001) Modulation of Kv1.5 potassium channel gating by extracellular zinc. Biophys J 81:125–136
Zhang S, Kwan DC, Fedida D, Kehl SJ (2001) External K+ relieves the block but not the gating shift caused by Zn2+ in human Kv1.5 potassium channels. J Physiol 532:349–358
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nigro, M.J., Perin, P. & Magistretti, J. Differential effects of Zn2+ on activation, deactivation, and inactivation kinetics in neuronal voltage-gated Na+ channels. Pflugers Arch - Eur J Physiol 462, 331–347 (2011). https://doi.org/10.1007/s00424-011-0972-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-011-0972-z