Abstract
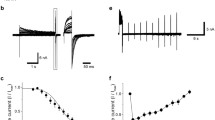
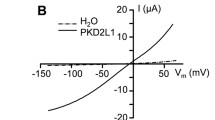
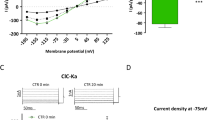
Polycystic kidney disease 2-like 1(PKD2L1), previously called transient receptor potential polycystin 3 (TRPP3), forms constitutively active voltage-dependent nonselective cation channels in the plasma membrane. The mechanism of regulation of PKD2L1 channels, however, has been poorly understood. In the present study, we found a bell-shaped alkaline pH dependence of PKD2L1 channel activity at the single-channel and whole-cell levels in patch-clamp recordings in HEK293T cells overexpressing mouse PKD2L1: alkalization to pH 8.0–9.0 increased the PKD2L1 currents, but alkalization to pH 10.0 decreased them. Single-channel analysis revealed that alkalization changed the open probability of PKD2L1 channels, but not their single-channel conductance. In addition, the voltage dependence of PKD2L1 channels was negatively and positively shifted by treatment with solutions of pH 8.0–9.0 and pH 10.0, respectively. These results indicate that the voltage-dependent gating of PKD2L1 channels was modulated by alkalization through two different mechanisms. Interestingly, we observed rebound activation of the PKD2L1 channel on washout of the alkaline solution after PKD2L1 channel inhibition at pH 10.0, suggesting that alkalization to pH 10.0 decreased PKD2L1 currents by inactivating the channels. Consistently, the PKD2L1 tail currents were accelerated by alkalization. These results suggest that alkalization is a bimodal modulator of mouse PKD2L1 channels.






Similar content being viewed by others
References
Basora N, Nomura H, Berger UV et al (2002) Tissue and cellular localization of a novel polycystic kidney disease-like gene product, polycystin-L. J Am Soc Nephrol 13:293–301
Chang RB, Waters H, Liman ER (2010) A proton current drives action potentials in genetically identified sour taste cells. Proc Natl Acad Sci USA 107:22320–22325
Chen XZ, Vassilev PM, Basora N et al (1999) Polycystin-L is a calcium-regulated cation channel permeable to calcium ions. Nature 401:383–386
Dhaka A, Uzzell V, Dubin AE et al (2009) TRPV1 is activated by both acidic and basic pH. J Neurosci 29:153–158
Fujita F, Uchida K, Moriyama T et al (2008) Intracellular alkalization causes pain sensation through activation of TRPA1 in mice. J Clin Invest 118:4049–4057
Huang AL, Chen X, Hoon MA et al (2006) The cells and logic for mammalian sour taste detection. Nature 442:934–938
Inada H, Kawabata F, Ishimaru Y et al (2008) Off-response property of an acid-activated cation channel complex PKD1L3-PKD2L1. EMBO Rep 9:690–697
Ishii S, Misaka T, Kishi M et al (2009) Acetic acid activates PKD1L3-PKD2L1 channel—a candidate sour taste receptor. Biochem Biophys Res Commun 385:346–350
Ishimaru Y, Inada H, Kubota M et al (2006) Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc Natl Acad Sci USA 103:12569–12574
Ishimaru Y, Katano Y, Yamamoto K et al (2010) Interaction between PKD1L3 and PKD2L1 through their transmembrane domains is required for localization of PKD2L1 at taste pores in taste cells of circumvallate and foliate papillae. FASEB J 24:4058–4067
Kawaguchi H, Yamanaka A, Uchida K et al (2010) Activation of polycystic kidney disease-2-like 1 (PKD2L1)-PKD1L3 complex by acid in mouse taste cells. J Biol Chem 285:17277–17281
Nelson TM, Lopezjimenez ND, Tessarollo L et al (2010) Taste function in mice with a targeted mutation of the pkd1l3 gene. Chem Senses 35:565–577
Nilius B, Talavera K, Owsianik G et al (2005) Gating of TRP channels: a voltage connection? J Physiol 567:35–44
Nilius B, Owsianik G, Voets T et al (2007) Transient receptor potential cation channels in disease. Physiol Rev 87:165–217
Nomura H, Turco AE, Pei Y et al (1998) Identification of PKDL, a novel polycystic kidney disease 2-like gene whose murine homologue is deleted in mice with kidney and retinal defects. J Biol Chem 273:25967–25973
Ramsey IS, Delling M, Clapham DE (2006) An introduction to TRP channels. Annu Rev Physiol 68:619–647
Ryu S, Liu B, Qin F (2003) Low pH potentiates both capsaicin binding and channel gating of VR1 receptors. J Gen Physiol 122:45–61
Shimizu T, Janssens A, Voets T et al (2009) Regulation of the murine TRPP3 channel by voltage, pH, and changes in cell volume. Pflügers Arch 457:795–807
Sugiura T, Tominaga M, Katsuya H et al (2002) Bradykinin lowers the threshold temperature for heat activation of vanilloid receptor 1. J Neurophysiol 88:544–548
Talavera K, Yasumatsu K, Voets T et al (2005) Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature 438:1022–1025
Tristani-Firouzi M, Sanguinetti MC (2003) Structural determinants and biophysical properties of HERG and KCNQ1 channel gating. J Mol Cell Cardiol 35:27–35
Vandenberg JI, Torres AM, Campbell TJ et al (2004) The HERG K+ channel: progress in understanding the molecular basis of its unusual gating kinetics. Eur Biophys J 33:89–97
Veldhuisen B, Spruit L, Dauwerse HG et al (1999) Genes homologous to the autosomal dominant polycystic kidney disease genes (PKD1 and PKD2). Eur J Hum Genet 7:860–872
Venkatachalam K, Montell C (2007) TRP channels. Annu Rev Biochem 76:387–417
Voets T, Droogmans G, Wissenbach U et al (2004) The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature 430:748–754
Voets T, Talavera K, Owsianik G et al (2005) Sensing with TRP channels. Nat Chem Biol 1:85–92
Wu G, Hayashi T, Park JH et al (1998) Identification of PKD2L, a human PKD2-related gene: tissue-specific expression and mapping to chromosome 10q25. Genomics 54:564–568
Wu LJ, Sweet TB, Clapham DE (2010) International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol Rev 62:381–404
Acknowledgments
We thank all members of the Toyama and Leuven laboratories for helpful discussions. We are grateful to Dr. Elbert L. Lee for careful reading of the manuscript. This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT) and from the Japan Society for the Promotion of Science (JSPS).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 90.4 kb)
Supplementary Fig. S1
(GIF 36.7 kb)
Supplementary Fig. S2
(GIF 28.6 kb)
Rights and permissions
About this article
Cite this article
Shimizu, T., Higuchi, T., Fujii, T. et al. Bimodal effect of alkalization on the polycystin transient receptor potential channel, PKD2L1. Pflugers Arch - Eur J Physiol 461, 507–513 (2011). https://doi.org/10.1007/s00424-011-0934-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-011-0934-5