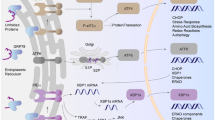
Abstract
The vascular endothelium plays a crucial role in vessel homeostasis and is implicated in the pathogenesis of cardiovascular disease. The function and life span of endothelial cells, therefore, have a large impact upon the quality and expectancy of an individual’s life. Exposure to haemodynamic forces determines the phenotype of endothelial cells. Turbulent blood flow, disturbed shear stress and a rising tension of the vessel wall result in endothelial dysfunction and an enhanced endothelial cell turnover. In this scenario, the role of endothelial mechanics is yet poorly described. The streaming blood exerts shear forces transmitted to the soft cortical actin mesh immediately underneath the plasma membrane. The mechanical properties of this actin cortex seem to be an important regulator of endothelial function. Aldosterone and high plasma sodium stiffen the endothelial cell cortex which is accompanied by a decrease in NO release. If endothelial stiffening is only transient, it may be a useful mechanism to compensate for any decrease in arterial blood pressure. Long-term stiffening of the cell, however, may lead to endothelial dysfunction and may contribute to cardiovascular disorders, as observed in disturbed aldosterone/sodium homeostasis. In this case, the mineralocorticoid receptor antagonist spironolactone maintains the endothelial cell cortex soft and thereby preserves normal endothelial function and longevity. This may explain the recently observed beneficial effects of spironolactone on the cardiovascular system. Taken together, the review highlights the importance of elasticity for normal endothelial function.


Similar content being viewed by others
References
Adrogue HJ, Madias NE (2007) Sodium and potassium in the pathogenesis of hypertension. N Engl J Med 356:1966–1978
Aird WC (2008) Endothelium in health and disease. Pharmacol Rep 60:139–143
Barrett-Connor E, Bush TL (1991) Estrogen and coronary heart disease in women. JAMA 265:1861–1867
Benetos A, Lacolley P, Safar ME (1997) Prevention of aortic fibrosis by spironolactone in spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol 17:1152–1156
Blacher J, Amah G, Girerd X, Kheder A, Ben Mais H, London GM, Safar ME (1997) Association between increased plasma levels of aldosterone and decreased systemic arterial compliance in subjects with essential hypertension. Am J Hypertens 10:1326–1334
Brilla CG, Matsubara LS, Weber KT (1993) Anti-aldosterone treatment and the prevention of myocardial fibrosis in primary and secondary hyperaldosteronism. J Mol Cell Cardiol 25:563–575
Brooks AR, Lelkes PI, Rubanyi GM (2002) Gene expression profiling of human aortic endothelial cells exposed to disturbed flow and steady laminar flow. Physiol Genomics 9:27–41
Cantiello HF, Stow JL, Prat AG, Ausiello DA (1991) Actin filaments regulate epithelial Na+ channel activity. Am J Physiol 261:C882–C888
Caplan BA, Schwartz CJ (1973) Increased endothelial cell turnover in areas of in vivo Evans Blue uptake in the pig aorta. Atherosclerosis 17:401–417
Chen BP, Li YS, Zhao Y, Chen KD, Li S, Lao J, Yuan S, Shyy JY, Chien S (2001) DNA microarray analysis of gene expression in endothelial cells in response to 24-h shear stress. Physiol Genomics 7:55–63
Davies PF (2009) Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med 6:16–26
Davies PF, Remuzzi A, Gordon EJ, Dewey CF Jr, Gimbrone MA Jr (1986) Turbulent fluid shear stress induces vascular endothelial cell turnover in vitro. Proc Natl Acad Sci USA 83:2114–2117
Davies PF, Zilberberg J, Helmke BP (2003) Spatial microstimuli in endothelial mechanosignaling. Circ Res 92:359–370
De Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MA Jr, Shin WS, Liao JK (1995) Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest 96:60–68
de Nigris F, Lerman LO, Ignarro SW, Sica G, Lerman A, Palinski W, Ignarro LJ, Napoli C (2003) Beneficial effects of antioxidants and l-arginine on oxidation-sensitive gene expression and endothelial NO synthase activity at sites of disturbed shear stress. Proc Natl Acad Sci USA 100:1420–1425
Dewey CF Jr, Bussolari SR, Gimbrone MA Jr, Davies PF (1981) The dynamic response of vascular endothelial cells to fluid shear stress. J Biomech Eng 103:177–185
Dimmeler S, Haendeler J, Nehls M, Zeiher AM (1997) Suppression of apoptosis by nitric oxide via inhibition of interleukin-1beta-converting enzyme (ICE)-like and cysteine protease protein (CPP)-32-like proteases. J Exp Med 185:601–607
Dimmeler S, Haendeler J, Zeiher AM (2002) Regulation of endothelial cell apoptosis in atherothrombosis. Curr Opin Lipidol 13:531–536
Florentin RA, Nam SC, Lee KT, Thomas WA (1969) Increased 3H-thymidine incorporation into endothelial cells of swine fed cholesterol for 3 days. Exp Mol Pathol 10:250–255
Foteinos G, Hu Y, Xiao Q, Metzler B, Xu Q (2008) Rapid endothelial turnover in atherosclerosis-prone areas coincides with stem cell repair in apolipoprotein E-deficient mice. Circulation 117:1856–1863
Fournet-Bourguignon MP, Castedo-Delrieu M, Bidouard JP, Leonce S, Saboureau D, Delescluse I, Vilaine JP, Vanhoutte PM (2000) Phenotypic and functional changes in regenerated porcine coronary endothelial cells: increased uptake of modified LDL and reduced production of NO. Circ Res 86:854–861
Funder JW (2005) Relative aldosterone excess: relative to what? Hypertension 46:643–644
Garg UC, Hassid A (1990) Nitric oxide-generating vasodilators inhibit mitogenesis and proliferation of BALB/C 3T3 fibroblasts by a cyclic GMP-independent mechanism. Biochem Biophys Res Commun 171:474–479
Hillebrand U, Hausberg M, Stock C, Shahin V, Nikova D, Riethmuller C, Kliche K, Ludwig T, Schillers H, Schneider SW, Oberleithner H (2006) 17beta-estradiol increases volume, apical surface and elasticity of human endothelium mediated by Na+/H+ exchange. Cardiovasc Res 69:916–924
Hobson B, Denekamp J (1984) Endothelial proliferation in tumours and normal tissues: continuous labelling studies. Br J Cancer 49:405–413
Hornsby PJ (2010) Senescence and life span. Pflugers Arch 459:291–299
Ingber DE (1997) Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol 59:575–599
Kasas S, Wang X, Hirling H, Marsault R, Huni B, Yersin A, Regazzi R, Grenningloh G, Riederer B, Forro L, Dietler G, Catsicas S (2005) Superficial and deep changes of cellular mechanical properties following cytoskeleton disassembly. Cell Motil Cytoskeleton 62:124–132
Kondrikov D, Han HR, Block ER, Su Y (2006) Growth and density-dependent regulation of NO synthase by the actin cytoskeleton in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 290:L41–L50
Kumar A, Meyerrose G, Sood V, Roongsritong C (2006) Diastolic heart failure in the elderly and the potential role of aldosterone antagonists. Drugs Aging 23:299–308
Kusche-Vihrog K, Sobczak K, Bangel N, Wilhelmi M, Nechyporuk-Zloy V, Schwab A, Schillers H, Oberleithner H (2008) Aldosterone and amiloride alter ENaC abundance in vascular endothelium. Pflugers Arch 455:849–857
Lombes M, Oblin ME, Gasc JM, Baulieu EE, Farman N, Bonvalet JP (1992) Immunohistochemical and biochemical evidence for a cardiovascular mineralocorticoid receptor. Circ Res 71:503–510
Maron BA, Leopold JA (2010) Aldosterone receptor antagonists: effective but often forgotten. Circulation 121:934–939
Mazzochi C, Bubien JK, Smith PR, Benos DJ (2006) The carboxyl terminus of the alpha-subunit of the amiloride-sensitive epithelial sodium channel binds to F-actin. J Biol Chem 281:6528–6538
Nerem RM, Levesque MJ, Cornhill JF (1981) Vascular endothelial morphology as an indicator of the pattern of blood flow. J Biomech Eng 103:172–176
Nishizaka MK, Zaman MA, Green SA, Renfroe KY, Calhoun DA (2004) Impaired endothelium-dependent flow-mediated vasodilation in hypertensive subjects with hyperaldosteronism. Circulation 109:2857–2861
Oberleithner H (2005) Aldosterone makes human endothelium stiff and vulnerable. Kidney Int 67:1680–1682
Oberleithner H, Riethmuller C, Ludwig T, Hausberg M, Schillers H (2006) Aldosterone remodels human endothelium. Acta Physiol (Oxf) 187:305–312
Oberleithner H, Riethmuller C, Schillers H, Macgregor GA, de Wardener HE, Hausberg M (2007) Plasma sodium stiffens vascular endothelium and reduces nitric oxide release. Proc Natl Acad Sci USA 104:16281–16286
Oberleithner H, Callies C, Kusche-Vihrog K, Schillers H, Shahin V, Riethmuller C, Macgregor GA, de Wardener HE (2009) Potassium softens vascular endothelium and increases nitric oxide release. Proc Natl Acad Sci USA 106:2829–2834
Oberleithner H, Kusche-Vihrog K, Schillers H (2010) Endothelial cells as vascular salt sensors. Kidney Int 77:490–494
Passerini AG, Polacek DC, Shi C, Francesco NM, Manduchi E, Grant GR, Pritchard WF, Powell S, Chang GY, Stoeckert CJ Jr, Davies PF (2004) Coexisting proinflammatory and antioxidative endothelial transcription profiles in a disturbed flow region of the adult porcine aorta. Proc Natl Acad Sci USA 101:2482–2487
Pesen D, Hoh JH (2005) Modes of remodeling in the cortical cytoskeleton of vascular endothelial cells. FEBS Lett 579:473–476
Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J (1999) The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 341:709–717
Prasain N, Stevens T (2009) The actin cytoskeleton in endothelial cell phenotypes. Microvasc Res 77:53–63
Radomski MW, Palmer RM, Moncada S (1987) Comparative pharmacology of endothelium-derived relaxing factor, nitric oxide and prostacyclin in platelets. Br J Pharmacol 92:181–187
Rajagopalan S, Duquaine D, King S, Pitt B, Patel P (2002) Mineralocorticoid receptor antagonism in experimental atherosclerosis. Circulation 105:2212–2216
Resnick N, Yahav H, Shay-Salit A, Shushy M, Schubert S, Zilberman LC, Wofovitz E (2003) Fluid shear stress and the vascular endothelium: for better and for worse. Prog Biophys Mol Biol 81:177–199
Ross R (1999) Atherosclerosis—an inflammatory disease. N Engl J Med 340:115–126
Rubanyi GM, Romero JC, Vanhoutte PM (1986) Flow-induced release of endothelium-derived relaxing factor. Am J Physiol 250:H1145–H1149
Sato M, Suzuki K, Ueki Y, Ohashi T (2007) Microelastic mapping of living endothelial cells exposed to shear stress in relation to three-dimensional distribution of actin filaments. Acta Biomater 3:311–319
Schiffrin EL (2006) Effects of aldosterone on the vasculature. Hypertension 47:312–318
Schnittler HJ, Schneider SW, Raifer H, Luo F, Dieterich P, Just I, Aktories K (2001) Role of actin filaments in endothelial cell–cell adhesion and membrane stability under fluid shear stress. Pflugers Arch 442:675–687
Schwartz SM (1978) Selection and characterization of bovine aortic endothelial cells. In Vitro 14:966–980
Schwartz SM, Benditt EP (1977) Aortic endothelial cell replication. I. Effects of age and hypertension in the rat. Circ Res 41:248–255
Schwartz SM, Gajdusek CM, Selden SC III (1981) Vascular wall growth control: the role of the endothelium. Arteriosclerosis 1:107–126
Serban DN, Nilius B, Vanhoutte PM (2010) The endothelial saga: the past, the present, the future. Pflugers Arch 459:787–792
Silvestre JS, Robert V, Heymes C, Aupetit-Faisant B, Mouas C, Moalic JM, Swynghedauw B, Delcayre C (1998) Myocardial production of aldosterone and corticosterone in the rat. Physiological regulation. J Biol Chem 273:4883–4891
Spaet TH, Lejnieks I (1967) Mitotic activity of rabbit blood vessels. Proc Soc Exp Biol Med 125:1197–1201
Su Y, Edwards-Bennett S, Bubb MR, Block ER (2003) Regulation of endothelial nitric oxide synthase by the actin cytoskeleton. Am J Physiol Cell Physiol 284:C1542–C1549
Takeda Y, Miyamori I, Yoneda T, Iki K, Hatakeyama H, Blair IA, Hsieh FY, Takeda R (1995) Production of aldosterone in isolated rat blood vessels. Hypertension 25:170–173
Taylor RG, Lewis JC (1986) Endothelial cell proliferation and monocyte adhesion to atherosclerotic lesions of white carneau pigeons. Am J Pathol 125:152–160
Tomaschitz A, Pilz S (2010) Aldosterone to renin ratio—a reliable screening tool for primary aldosteronism? Horm Metab Res 42:382–391
Vanhoutte PM (2010) Regeneration of the endothelium in vascular injury. Cardiovasc Drugs Ther 24:299–303
Wagner AH, Guldenzoph B, Lienenluke B, Hecker M (2004) CD154/CD40-mediated expression of CD154 in endothelial cells: consequences for endothelial cell–monocyte interaction. Arterioscler Thromb Vasc Biol 24:715–720
Wang N, Tolic-Norrelykke IM, Chen J, Mijailovich SM, Butler JP, Fredberg JJ, Stamenovic D (2002) Cell prestress. I. Stiffness and prestress are closely associated in adherent contractile cells. Am J Physiol Cell Physiol 282:C606–C616
Weinbaum S, Zhang X, Han Y, Vink H, Cowin SC (2003) Mechanotransduction and flow across the endothelial glycocalyx. Proc Natl Acad Sci USA 100:7988–7995
Williams TA, Verhovez A, Milan A, Veglio F, Mulatero P (2006) Protective effect of spironolactone on endothelial cell apoptosis. Endocrinology 147:2496–2505
Wong AJ, Pollard TD, Herman IM (1983) Actin filament stress fibers in vascular endothelial cells in vivo. Science 219:867–869
Xu Q (2009) Disturbed flow-enhanced endothelial turnover in atherosclerosis. Trends Cardiovasc Med 19:191–195
Yoder MC (2010) Is endothelium the origin of endothelial progenitor cells? Arterioscler Thromb Vasc Biol 30:1094–1103
Acknowledgements
We thank Prof. Hugh E. de Wardener, Imperial College, London, for critically reading the manuscript. This work was supported by the Deutsche Forschungsgemeinschaft (OB63/17-1, Koselleck OB 63/18).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kliche, K., Jeggle, P., Pavenstädt, H. et al. Role of cellular mechanics in the function and life span of vascular endothelium. Pflugers Arch - Eur J Physiol 462, 209–217 (2011). https://doi.org/10.1007/s00424-011-0929-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-011-0929-2