Abstract
Transient receptor potential V3 (TRPV3) and TRPV4 are heat-activated cation channels expressed in keratinocytes. It has been proposed that heat-activation of TRPV3 and/or TRPV4 in the skin may release diffusible molecules which would then activate termini of neighboring dorsal root ganglion (DRG) neurons. Here we show that adenosine triphosphate (ATP) is such a candidate molecule released from keratinocytes upon heating in the co-culture systems. Using TRPV1-deficient DRG neurons, we found that increase in cytosolic Ca2+-concentration in DRG neurons upon heating was observed only when neurons were co-cultured with keratinocytes, and this increase was blocked by P2 purinoreceptor antagonists, PPADS and suramin. In a co-culture of keratinocytes with HEK293 cells (transfected with P2X2 cDNA to serve as a bio-sensor), we observed that heat-activated keratinocytes secretes ATP, and that ATP release is compromised in keratinocytes from TRPV3-deficient mice. This study provides evidence that ATP is a messenger molecule for mainly TRPV3-mediated thermotransduction in skin.
Similar content being viewed by others
Introduction
Thermal information detected by the skin layers has to be converted to electrical signals, which then is transmitted to the central nervous system. It is thought that temperature-activated receptors expressed in sensory processes within the skin are directly activated by changes in temperature. For example, capsaicin receptor TRPV1 and menthol receptor TRPM8 expressed in the sensory nerve endings cause depolarization by cation influx through the channels in response to hot and cold stimuli, respectively, followed by action potential generation [5, 27, 32]. These thermosensitive transient receptor potential (TRP) channels belong to the TRP ion channel super family, which consists of six related protein families in mammals [10, 34, 38]. In addition to TRPV1 and TRPM8, seven TRP channels are shown to be involved in thermosensation with distinct temperature thresholds. In contrast to the four heat- or cold-sensitive TRPV1, TRPV2, TRPM8, and TRPA1, two TRPV (TRPV3 and TRPV4) and three TRPM (TRPM2, TRPM4, and TRPM5) channels have been found to be activated by warm stimuli [10, 13, 38, 39]. Interestingly, TRPV3 and TRPV4 are predominantly expressed in skin keratinocytes, suggesting that warm stimuli can be initially detected in skin [7, 10, 13, 33, 38]. Indeed, mice lacking TRPV3 or TRPV4 have been reported to exhibit abnormal thermo-related behaviors [25, 28].
Intercellular signaling mediated by purines is present early in evolution and, therefore, is a widespread route for cell-to-cell communication. The concept of purinergic transmission has been well accepted since the report showing that adenosine triphosphate (ATP) is a transmitter in non-adrenergic, non-cholinergic inhibitory nerves [3]. Now it is recognized that ATP acts as either sole transmitter or a co-transmitter in most nerves in both the peripheral nervous system and central nervous system [4]. The epidermis is extensively innervated by axons arising from peripheral sensory neurons, which convey sensory input to the central nervous system. These sensory afferents are intimately associated with keratinocytes, mast cells, and Langerhans cells, indicating the capacity of peripheral sensory endings to monitor the status of the skin [2]. Signaling processes in keratinocytes have been investigated largely from the aspect of mechanisms such as differentiation and metabolism. However, several lines of evidence indicate a complex system of communication between skin cells and the sensory afferents innervating the skin [10, 14, 26, 38].
The concept of skin keratinocytes initially detecting ambient temperatures indicates that temperature information has to be transmitted to adjacent sensory neurons [13, 24, 31]. Electron microscopic studies have not identified synapse-like structures between keratinocytes and sensory nerve endings [6], raising the possibility that some diffusible molecules can function like neurotransmitters between keratinocytes and sensory nerve endings. However, no such evidence has been provided. In this study, we identified the candidate molecule, ATP, transferring temperature information from keratinocytes to sensory neurons by establishing a co-culture system.
Materials and methods
Animals
Neonatal (P2) wild-type (C57BL/6-strain, SLC, Shizuoka, Japan), TRPV1-deficient (from Dr. Julius D), TRPV3-deficient (from Dr. Patapoutian A), or TRPV4-deficient mice (from Dr. Suzuki M) were housed in a controlled environment. All the procedures were in accordance with our institute (National Institute of physiological Sciences) and the National Institute of Health guides for the care and use of laboratory animals.
DRG neuron and keratinocyte cultures from P2 mice
Dorsal root ganglion (DRG) neurons were isolated as previously described [29]. Freshly isolated neurons were plated onto cover slips and cultured in keratinocyte medium with added 100 ng/ml 7S nerve growth factor at 33°C with 5% CO2 for 2 days. Keratinocytes were obtained by modifying earlier described method [7]. Briefly, keratinocytes from P2 mice were cultured in MCDB keratinocyte medium (Sigma, St. Louis, MO) containing (mg/ml) 5 insulin, 0.4 hydrocortisone, 10 transferrin, 14.1 phosphorylethanolamine, 0.01 EGF, 25 gentamicin, 50 streptomycin, and 40 bovine pituitary gland extract (BPE; Kyokuto, Tokyo, Japan) with 50 U/ml penicillin and 1% fetal bovine serum (FBS). Cells, 5 × 104, plated on cover slips were incubated over night in MCDB keratinocyte medium, before replacing with FBS-free MCDB medium containing BPE and CaCl2 (0.04 mM). Cells were differentiated in FBS-free MCDB medium containing BPE and 1.2 mM CaCl2 for 24 h at 33°C and 5% CO2. For keratinocytes in co-culture with DRG neurons, MCDB was replaced with keratinocyte medium [7].
Co-culture of HEK293 cells and keratinocytes
Human embryonic kidney-derived 293 (HEK293) cells maintained in DMEM with FBS/penicillin/streptomycin were co-transfected with 1 µg rat P2X2 or 1 µg mouse 5HT3A and 0.1 µg of pGREEN LANTAN 1 cDNAs, using Lipofectamine Plus reagent (Invitrogen, Carlsbad, CA, USA) for ATP or serotonin bio-sensor assays, respectively. For a glutamate bio-sensor assay, HEK293 cells were co-transfected with 0.5 µg mouse NR1/0.5 µg mouse NR2B and 0.1 µg of pGREEN LANTAN 1 cDNAs in Basal Medium Eagle (BME; Sigma). Following transfection, HEK293 cells were re-plated to be co-cultured with keratinocytes in MCDB medium containing BPE and 1.2 mM CaCl2. Glutamate-induced cytotoxicity to NR1/NR2B expressing HEK293 cells in co-culture with keratinocytes was overcome by adding ketamine (Sigma, 500 µM) and D-AP5 (Sigma, 250 µM) to medium.
Immunocytochemistry
DRG neuron and keratinocyte co-cultures were triple-immunostained with primary antibodies; mouse monoclonal anti-β tubulin (Sigma) for DRG neurons, rabbit polyclonal anti-MK14 (Covance, Berkeley, CA) for basal keratinocytes, and 4',6-diamidino-2-phenylindole (DAPI; Dojindo, Kumamoto, Japan) for nuclei. Secondary antibodies included Alexa Fluor 546 anti-mouse IgG and Alexa Fluor 488 anti-rabbit IgG (Molecular probes, Eugene, OR, USA).
Ca2+-imagings using fura-2 or fluo-4
Fura-2 or fluo-4 fluorescence was measured in the bath solution containing (mM) 140 NaCl, 5 KCl, 2 CaCl2, 2 MgCl2, 10 HEPES, and 10 glucose (pH 7.4) using a Nikon microscope (Nikon, Tokyo, Japan) connected to a Hamaphoto CCD camera (Hamamatsu Photonics, Shizuoka, Japan) and represented as 340/380 nm ratio for fura-2 or F/Fbackground for fluo-4, respectively. Heating in the experiments with fluo-4 was done by using a microscope stage heater (PH-1 and TC-344B, Warner Instruments, Hamden, CT, USA) to avoid washout of released ATP from keratinocytes. Heating in the experiments with fura-2 was done by using an inline-heater (SH-27B and TC-344B, Warner Instruments). A thermocouple (TA-30, Warner Instruments) was used to measure temperature.
Electrophysiology
Whole-cell patch-clamp recordings and sampling from transfected HEK293 cells in co-culture with keratinocytes were carried out as described before [5]. Bath solution was the same as in the Ca2+-imaging, and pipette solution contains (mM) 140 KCl, 5 EGTA, 10 HEPES, pH 7.4 (adjusted with KOH). Voltage-ramps (−100 to +100 mV with 5-s intervals) were applied. In a glutamate bio-sensor assay, magnesium-free bath solution and CsCl pipette solution were used. Heating was done by using a microscope stage heater.
Measurement of ATP release
The mouse scrotal skin was used. The mouse was anesthetized with pentobarbital sodium (70 mg/kg, i.p.) and hairs of scrotum were removed by depilator cream. The scrotal skin was then removed under surgically clean condition, and put in a small chamber (volume: 1.5 ml) and superfused 1.5 ml/min with sterilized Krebs–Henseleit solution (containing (in mM) 110.9 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 25.0 NaHCO3, and 20 glucose) continuously bubbled with a gas mixture of 95% O2 and 5% CO2. In half the cases, ruthenium red (RR, 50 µM) was applied to the superfusate, and experiments were started after pre-incubation for >20 min. Temperature in the chamber was continuously monitored near the orifice of the sampling tube by a thermocouple (Physitemp, Clifton, USA) and kept constant (either 24.9 ± 0.0°C). Before warm stimulation at least 1 h was elapsed. The superfusate was sampled at 0.8 ml/min for 15 s using a peristaltic pump through plastic tubing with a metal tube (2 mm in diameter) at one end. The tip of the metal tube was placed near the orifice of overflow opposite to the inflow. The delay time (15 s) passing through this piping was compensated later for analysis. The superfusate was sampled every 15 s for 2.5 min during the period of steep temperature change and before and after that 1–5-min intervals. Temperature was increased up to 41.9 ± 0.2°C in about 1 min by changing temperature of the water circulating outside the bath and inlet tubing. The ATP concentration was measured with luciferin–luciferase method. Briefly, 100 µl aliquot of the bath sample was added to 100 µl of ATP assay mix (Enliten ATP assay system, Promega, Madison, WI, USA) and the luminescence was measured with a luminometer (Lumat LB 9507, Berthold Technologies, Germany) for 10 s. Net ATP release in the sample was calculated by subtracting the basal concentration measured before stimulation from that induced by the thermal stimulation. The surface area of the skin was measured afterward, and the ATP release was normalized to that of 100 mm2.
Cell death assay
Same amounts of wild-type keratinocytes co-cultured with TRPV1-deficient DRG neurons were treated without or with heat. Then, propidium iodide (PI) was added to detect the dead cells in the dishes. Numbers of PI-positive dead cells were counted in glass cover slips (12 cover slips each).
Data analysis
Values are shown as mean ± SEM. p values <0.05 were considered significant.
Results
Intracellular Ca2+ increase in sensory neurons secondary to the one in keratinocytes involves ATP signals
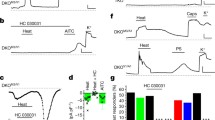
We first examined changes in intracellular Ca2+ levels ([Ca2+]i) of DRG neurons from wild-type (WT) and TRPV1-deficient (TRPV1KO) mice. We found that heat application (∼45°C) evoked increases in [Ca2+]i in all capsaicin-sensitive neurons (0.74 ± 0.03, n = 190; Supplemental Fig. 1a, c). However, no such response was observed in DRG neurons from TRPV1KO mice (0.08 ± 0.01, n = 220; Supplemental Fig. 1b, c) while DRG neurons from both WT and TRPV1KO mice responded to ATP similarly, indicating an unaltered purinergic system in the TRPV1-deficient DRG neurons (Supplemental Fig. 1a, b). This result prompted us to establish a co-culture of keratinocytes and DRG neurons from TRPV1KO mice to avoid the cell-autonomous TRPV1-mediated heat responses in DRG neurons. Immunostaining of the co-culture with antibodies showed close proximity of neuronal terminals and keratinocytes (Fig. 1a). To obtain a sensitive readout of the signaling between such keratinocytes and DRG neurons in close proximity, the single wavelength-excitation Ca2+-indicating dye, fluo-4, was used to capture responses less than a second time frame. To avoid the washout of candidate molecules released from keratinocytes upon heating, we used a stage heater without solution flow. Nonspecific reduction of fluo-4 ratio was observed upon heating (Fig. 1b, c, d) because of temperature-dependent change of the signals. We found that heat application (∼40°C) to DRG neuron-keratinocyte co-culture from TRPV1KO mice evoked a [Ca2+]i increase in both keratinocytes and DRG neurons as shown in Fig. 1a (n = 244, Fig. 1b), while neurons with their terminals not in close proximity of keratinocytes did not show such heat-evoked [Ca2+]i increase (data not shown). The heat-induced [Ca2+]i increase in a DRG neuron cell body was delayed (1.21 ± 0.17 s, n = 8) compared to that in a keratinocyte, lying in close proximity to the axon terminal originating from the neuron cell body (Fig. 1b), suggesting that the DRG neuron response was secondary to the keratinocyte response. Both a DRG neuron and a keratinocyte responded to ATP (100 µM) with a similar time course (Fig. 1b). Pretreatment of the co-culture with global P2 receptor antagonists, pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonate (PPADS; 100 µM) or suramin (100 µM), completely inhibited the heat-induced [Ca2+]i increase in the DRG neuron cell bodies, but not in the keratinocytes (n = 270 with PPADS in Fig. 1c, and n = 43 with suramin in Fig. 1d). On the other hand, ATP application (100 µM) showed subdued purinergic responses in both cell types in the experiments with PPADS, possibly due to incomplete washout of the compound (Fig. 1c). Similar results were obtained in the co-culture of DRG neurons and keratinocytes from wild-type mice (data not shown). We confirmed that heat stimulus did not cause cell death in these experiments (Supplemental Fig. 2). These results suggest that ATP is responsible for the transmission of the heat stimulus from keratinocytes to sensory neurons in culture.
Cytosolic Ca2+ increase in DRG neurons upon heating was secondary to that in adjacent keratinocytes via ATP. a A representative co-culture showing the proximity between DRG neurons and keratinocytes from TRPV1-deficient mice, immunostained with antibodies; anti-β-tubulin III for DRG (red), anti-MK14 for keratinocytes (green) and DAPI for nuclei (blue; n = 8). b A representative trace showing heat application (a black trace), evoked a rise in intracellular Ca2+ levels in a DRG neuron cell body (a red trace) secondary to that in a keratinocyte (a green trace). Dotted black lines represent the time difference (1.21 ± 0.17 s) in commencement of response between a keratinocyte and a DRG neuron cell body. Further application of ATP (100 µM) confirmed purinergic responses in both cell types. Traces are representative of typical experiments (N = 33 experiments). The neurons with nerve terminals in close proximity of keratinocytes responded to heat stimulus (n = 244). An inset is expanded from the square box. Flow was stopped during heating to avoid washout of the candidate molecules released from keratinocytes. c, d A representative trace showing that a 5-min pretreatment with PPADS (100 µM, c) or treatment with suramin (100 µM, d) blocked heat-induced response in a DRG neuron (a red trace) but not in a keratinocyte (green trace) in close proximity. Further application of ATP (100 µM) confirmed purinergic responses in both cell types. Traces are representative of typical experiments (n = 27 with PPADS and n = 43 with suramin)
Evidence of ATP release from keratinocytes via predominant TRPV3 activation upon heating in the bio-sensor system
We then tested directly whether keratinocytes secrete ATP upon heating. In order to detect the local ATP released within the microenvironment of warmed keratinocytes, we established a bio-sensor system [17]. In this system, ionotropic receptors expressed in HEK293 cells placed closely to keratinocytes detect the molecules released from keratinocytes upon stimulus (Fig. 2a). During heat stimulus (∼40°C) to the co-culture of wild-type keratinocytes with HEK293 cells expressing P2X2 receptors, P2X2-like inwardly rectifying currents were recorded from the whole-cell patch-clamped HEK293 cells only when placed in close proximity to the keratinocytes (less than 10 µm in the distance; Fig. 2b, n = 4), suggesting ATP release from the keratinocytes upon heating. Functional expression of P2X2 receptors in the HEK293 cell was confirmed by ATP (100 µM) application in the condition with two cell types apart to avoid possible effect of ATP-induced ATP release from keratinocytes (Fig. 2b). Similar P2X2 receptor-mediated currents in the HEK293 cells were observed in the co-culture with keratinocytes from TRPV1KO mice (Fig. 2c, n = 5), confirming the results shown in Fig. 1. Because TRPV3 and TRPV4 are possible candidates for heat detectors in keratinocytes, heat-evoked P2X2 receptor-mediated currents were examined in the co-culture with keratinocytes from either TRPV4-deficient (TRPV4KO, Fig. 2d) or TRPV3-deficient (TRPV3KO, Fig. 2e) mice. Heat-evoked P2X2 receptor-mediated currents in the co-culture with keratinocytes from TRPV3KO mice were significantly smaller (0.15 ± 0.02 pA/pF, n = 7) than those in the co-culture with keratinocytes from WT (0.43 ± 0.02 pA/pF, n = 4), TRPV1KO (0.36 ± 0.04 pA/pF, n = 5), or TRPV4KO (0.39 ± 0.07 pA/pF, n = 7) mice (Fig. 2f, p < 0.05). These results suggest that TRPV3 is predominantly required for heat-induced ATP release. A semi-quantitative estimate for the amount of heat-induced ATP release from keratinocytes was obtained by equating the heat-evoked P2X2 receptor-mediated currents in the co-culture with ATP-evoked P2X2 receptor-mediated currents not in co-culture (Supplemental Fig. 3a). This comparison resulted in an estimated release of about 1 µM ATP from the TRPV3KO keratinocytes and about 10 µM ATP from other keratinocytes. In addition, the Ca2+-imaging experiments showed that the percentage of keratinocytes from TRPV3KO mice responding to heat (26.6 ± 4.4%, 179/640 cells) was significantly lower (p < 0.005) than those from WT (76.4 ± 2.7%, 538/708 cells), TRPV1KO (79.1 ± 2.9%, 507/648 cells) or TRPV4KO (77.3 ± 5.4%, 567/611 cells) mice (Supplemental Fig. 3b), consistent with the data shown in Fig. 2f. We examined the involvement of TRPV3 or TRPV4 in the ATP release from keratinocytes in the co-culture system by applying the ligand, camphor (5 mM), or 4α-PDD (1 µM), respectively. Because TRPV1 has been reported to be activated by camphor [42] and to be expressed in keratinocytes [12], we used TRPV1-deficient keratinocytes in the co-culture for the experiment with camphor. To avoid the washout of molecules released from keratinocytes upon stimulus, we applied the agonists for 20 s and stopped the flow. Both camphor (n = 5) and 4α-PDD (n = 5) could cause activation of P2X2 channels expressed in HEK293 cells in the system (Fig. 3a, b) although time to activation of P2X2-mediated currents varied because of the stopped flow and the fact that it requires 1–2 min for 4α-PDD to activate the TRPV4 in heterologous expression system [40]. These results suggest that activation of both TRPV3 and TRPV4 causes ATP release from keratinocytes, a little different from the results in Fig. 2.
P2X2-mediated currents were observed upon heating in HEK293 cells expressing P2X2 co-cultured with mouse keratinocytes. a Cartoons showing a bio-sensor system. HEK293 cells expressing bio-sensors were placed very close to the heated keratinocytes, then the ability of the bio-sensors to detect the molecules were confirmed by applying their agonists away from the keratinocytes. b–e Representative traces showing that heat application evoked whole-cell current responses (the left panels) with inward rectification (green traces in the right panels) in the HEK293 cells transfected with P2X2 cDNA in co-culture with keratinocytes from wild-type (WT, b), TRPV1-deficient (TRPV1KO, c), TRPV4-deficient (TRPV4KO, d) or TRPV3-deficient (TRPV3KO, e) mice. Further application of ATP (100 µM) was used to confirm P2X2-mediated responses (the left panels) with inward rectification (blue traces in the right panels). A ramp-pulse (from −100 mV to +100 mV for 500 ms) was applied with 5-s intervals. Holding potential was −60 mV. Traces are representative of typical experiments (N = 4–7). f Heat-induced P2X2-mediated currents were normalized to the responses evoked by 100 µM ATP from four to seven typical experiments (b–e). Parenthesis indicate the numbers of cells tested. *p < 0.05, student t test
Camphor and 4α-PDD response in keratinocytes. a, b Representative whole-cell current traces in P2X2-expressing HEK293 cells co-cultured with TRPV1-deficient (TRPV1KO; a) or wild-type (b) keratinocytes upon application of camphor (5 mM, a) or 4α-PDD (1 µM, b). Similar results were observed in other five cells tested
Serotonin or glutamate was not released from keratinocytes upon heating
We also tested if other candidate molecules are secreted from keratinocytes upon heating. We used additional bio-sensor systems to detect serotonin or glutamate release from keratinocytes upon heating (∼40°C). We looked for serotonin, since this transmitter has been reported to be involved in taste signal transmission from taste cells to afferent neurons [35], although ATP has been recently shown to transmit taste information between taste cells and gustatory nerves [15]. We also looked for glutamate since glutamate release from Merkel cells in skin is known to be associated with mechanotransduction [16]. To detect heat-induced release of serotonin or glutamate from keratinocytes, HEK293 cells expressing the serotonin receptor, 5HT3A, or the glutamate receptor subunits, NR1/NR2B, were co-cultured with keratinocytes from wild-type mice. No serotonin- (n = 10) or glutamate- (n = 10) evoked currents were observed in HEK293 cells in close proximity to keratinocytes exposed to heat stimulus (Fig. 4a, b). Functional expression of 5HT3A or NR1/NR2B receptors in the patch-clamped HEK293 cells was confirmed by applying serotonin (100 µM; Fig. 4a) or a combination of glutamate (50 µM) with glycine (10 µM; Fig. 4b), respectively, in a condition where the two cell types were apart to avoid possible effect of serotonin- or glutamate-induced signaling from keratinocytes.
5HT3A- nor NMDA-receptor-mediated responses were observed in HEK293 cells upon heating. a, b There was no 5HT3A-mediated (a) or NMDA-receptor-mediated (b) responses upon heat application to the co-culture while control inward currents with typical rectifications were observed in response to serotonin (100 µM; a) or a combination of glutamate (50 µM) + glycine (10 µM; b). Traces are representative of typical experiments (n = 10)
ATP-evoked current activation upon heating in the co-culture of keratinocytes and sensory neurons
In order to confirm ATP-mediated signal transmission to sensory neurons from the keratinocytes upon heating, we performed patch-clamp experiments using DRG neurons and keratinocytes from TRPV1KO mice. We added acutely dissociated DRG neurons to the keratinocyte culture because whole-cell patch-clamped DRG neurons had to be lifted up and moved close to the keratinocytes. We observed transient inward currents (0.33 ± 0.11 nA, n = 9) in the small DRG neurons (cell capacitance 7.5 ± 0.5 pF) in close proximity to the heated keratinocytes (Fig. 5a). ATP (100 µM) also activated transient inward currents, indicative of the opening of P2X3 channels. We also heated TRPV3KO keratinocytes co-cultured with TRPV1KO DRG neurons (n = 18). However, no significant ATP-activated current responses were obtained (Fig. 5b), supporting the predominant involvement of TRPV3 in heat-evoked ATP release shown in Fig. 2.
Heat-evoked ATP-mediated currents in DRG neurons with TRPV1-deficient keratinocytes, but not with TRPV3-deficient keratinocytes. Representative traces showing that heat application evoke a current response in a DRG neuron with keratinocytes from TRPV1-deficient (TRPV1KO) mice (a, n = 9), but not from TRPV3-deficient (TRPV3KO) mice (b, n = 18)
Measurement ATP release upon heating from skin in the skin-nerve preparation
Finally, we assessed whether heat-evoked ATP release is observed in tissue level. The excised mouse scrotal skin, where excellent warm-sensitivity and warm-sensitive fibers have been reported [18, 21], was superfused in a small bath, and ATP concentration in the bath solution was measured by luciferin–luciferase assay. Upon heat application (∼42°C), temperature-dependent increase of ATP concentration was observed (Fig. 6, blue). This heat-induced ATP release was significantly reduced by ruthenium red (RR, 50 µM), a broad TRP channel inhibitor (Fig. 6, red) at higher temperature range: in the presence of ruthenium red, the RR-insensitive ATP release activated at lower temperature range (>∼25°C) was still observed. However, RR-sensitive fraction of ATP release was apparent first at ∼40°C (Fig. 6, at 1 min; temperature range of the fraction, 40.3–41.9°C), showing a good agreement with the temperature needed for ATP release from keratinocytes and activation of TRPV3- or TRPV4-expressing HEK293 cells. These results support that TRPV3 and TRPV4 would be involved in heat-evoked ATP release in the skin.
Heat-evoked ATP release from mouse scrotal skin in the skin-nerve preparation. Heat application (a black line, n = 18) caused ATP release from the isolated mouse scrotal skin in the normal bath solution (control, blue circles, n = 9). The response was decreased in the presence of ruthenium red (50 µM started at −5 min, red diamonds, n = 9). The ATP level was plotted at the middle of each sampling period (15 s). *p < 0.05, vs. control by Mann–Whitney test
Discussion
We showed heat-induced ATP release from keratinocytes and that purinoreceptor antagonists blocked the heat-activated communication between the skin and nerve in the Ca2+-imaging. Furthermore, small amount of local ATP, but not serotonin or glutamate released from keratinocytes upon heating could be detected using a patch-clamp-based bio-sensor system with ionotropic ATP receptors expressed in HEK293 cells. This bio-sensor system could be used for detecting many other molecules locally released from not only keratinocytes but also other cell types including neurons and glias. The involvement of ATP in physiological signal transmission in thermosensation clarified in this study is consistent with the well-documented concept of sensory afferent signaling via purinergic nerves innervating various tissues [9, 22, 23], the report that P2X3-deficient mice exhibited warm-coding deficits [36], and that warm temperature sensation has been proposed to involve fast communication between the skin and CNS via the DRG [10, 13, 24, 31, 38].
Our data suggests that TRPV3 mainly mediates the release of ATP from keratinocytes upon heating, being consistent with the recent report showing that keratinocytes participate in thermal pain transduction through TRPV3 in keratinocytes although PGE2 is an intercellular messenger [19]. Our results are also agreement with that DS-Nh mice having TRPV3 gain-of-function mutation exhibited higher Ca2+ influx upon thermal stimulation in the epidermal sheet preparation [20, 30]. However, we cannot rule out contributions by TRPV4. Since 4α-PDD caused activation of P2X2 channels to the extent comparable to that evoked by 100 µM of ATP (Fig. 3b), and since heat-evoked P2X2-mediated current responses were not completely abolished in our bio-sensor system using TRPV3KO keratinocytes (Fig. 2e). These data suggest the existence of other thermo-sensors in vivo. The results that none of 18 TRPV1KO DRG neurons showed P2X-like current responses upon heating of keratinocytes (Fig. 5) contradict the residual response observed in the co-culture with TRPV3KO keratinocytes (Fig. 2e). This is partially because the mechanisms connecting TRP channel activation and ATP release are largely unknown. In neuronal cells, depolarizing stimulation resulted in exocytotic release of ATP in hippocampal slices [41]. However, in non-excitable cells including HEK293 cells, the mechanism of ATP release is still a matter of debate. In astrocytes, there have been several reports that ATP can be released via chloride channels [11], gap junction hemi-channels [37], ATP-binding cassette [1], and exocytosis [8] some of which require [Ca2+]i increase. Keratinocytes and astrocytes might share the same mechanism for the ATP release. In any case, ATP release from keratinocytes could potentially signal to a variety of P2X- or P2Y-expressing sensory terminals within the epidermis to transmit innocuous temperature information; however, a role in noxious heat transmission cannot be ruled out.
References
Ballerini P, Di Iorio P, Ciccarelli R, Nargi E, D'Alimonte I, Traversa U, Rathbone MP, Caciagli F (2002) Glial cells express multiple ATP binding cassette proteins which are involved in ATP release. Neuroreport 13:1789–1792
Boulais N, Misery L (2008) The epidermis: a sensory tissue. Eur J Dermatol 18:119–127
Burnstock G (1972) Purinergic nerves. Pharmacol Rev 24:509–581
Burnstock G (2007) Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 87:659–797
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389:816–824
Chateau Y, Misery L (2004) Connections between nerve endings and epidermal cells: are they synapses? Exp Dermatol 13:2–4
Chung MK, Lee H, Mizuno A, Suzuki M, Caterina MJ (2004) TRPV3 and TRPV4 mediate warmth-evoked currents in primary mouse keratinocytes. J Biol Chem 279:21569–21575
Coco S, Calegari F, Pravettoni E, Pozzi D, Taverna E, Rosa P, Matteoli M, Verderio C (2003) Storage and release of ATP from astrocytes in culture. J Biol Chem 278:1354–1362
Cook SP, McCleskey EW (2002) Cell damage excites nociceptors through release of cytosolic ATP. Pain 95:41–47
Damann N, Voets T, Nilius B (2008) TRPs in our senses. Curr Biol 18:R880–R889
Darby M, Kuzmiski JB, Panenka W, Feighan D, MacVicar BA (2003) ATP released from astrocytes during swelling activates chloride channels. J Neurophysiol 89:1870–1877
Denda M, Fuziwara S, Inoue K, Denda S, Akamatsu H, Tomitaka A, Matsunaga K (2001) Immunoreactivity of VR1 on epidermal keratinocyte of human skin. Biochem Biophys Res Commun 285:1250–1252
Dhaka A, Viswanath V, Patapoutian A (2006) Trp ion channels and temperature sensation. Annu Rev Neurosci 29:135–161
Dussor G, Koerber HR, Oaklander AL, Rice FL, Molliver DC (2009) Nucleotide signaling and cutaneous mechanisms of pain transduction. Brain research reviews 60:24–35
Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC (2005) ATP signaling is crucial for communication from taste buds to gustatory nerves. Science 310:1495–1499
Haeberle H, Fujiwara M, Chuang J, Medina MM, Panditrao MV, Bechstedt S, Howard J, Lumpkin EA (2004) Molecular profiling reveals synaptic release machinery in Merkel cells. Proc Natl Acad Sci U S A 101:14503–14508
Hayashi S, Hazama A, Dutta AK, Sabirov RZ, Okada Y (2004) Detecting ATP release by a biosensor method. Sci STKE 2004:pl14
Hellon RF, Hensel H, Schafer K (1975) Thermal receptors in the scrotum of the rat. J Physiol 248:349–357
Huang SM, Lee H, Chung MK, Park U, Yu YY, Bradshaw HB, Coulombe PA, Walker JM, Caterina MJ (2008) Overexpressed transient receptor potential vanilloid 3 ion channels in skin keratinocytes modulate pain sensitivity via prostaglandin E2. J Neurosci 28:13727–13737
Imura K, Yoshioka T, Hikita I, Tsukahara K, Hirasawa T, Higashino K, Gahara Y, Arimura A, Sakata T (2007) Influence of TRPV3 mutation on hair growth cycle in mice. Biochem Biophys Res Commun 363:479–483
Ishikawa Y, Nakayama T, Kanosue K, Matsumura K (1984) Activation of central warm-sensitive neurons and the tail vasomotor response in rats during brain and scrotal thermal stimulation. Pflugers Arch 400:222–227
Khakh BS, North RA (2006) P2X receptors as cell-surface ATP sensors in health and disease. Nature 442:527–532
Koizumi S, Fujishita K, Inoue K, Shigemoto-Mogami Y, Tsuda M (2004) Ca2+ waves in keratinocytes are transmitted to sensory neurons: the involvement of extracellular ATP and P2Y2 receptor activation. Biochem J 380:329–338
Lee H, Caterina MJ (2005) TRPV channels as thermosensory receptors in epithelial cells. Pflugers Arch 451:160–167
Lee H, Iida T, Mizuno A, Suzuki M, Caterina MJ (2005) Altered thermal selection behavior in mice lacking transient receptor potential vanilloid 4. J Neurosci 25:1304–1310
Lumpkin EA, Caterina MJ (2007) Mechanisms of sensory transduction in the skin. Nature 445:858–865
McKemy DD, Neuhausser WM, Julius D (2002) Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416:52–58
Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, Andahazy M, Story GM, Patapoutian A (2005) Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science 307:1468–1472
Moriyama T, Higashi T, Togashi K, Iida T, Segi E, Sugimoto Y, Tominaga T, Narumiya S, Tominaga M (2005) Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Molecular Pain 1:3–12
Nilius B, Owsianik G, Voets T, Peters JA (2007) Transient receptor potential cation channels in disease. Physiol Rev 87:165–217
Patapoutian A, Peier AM, Story GM, Viswanath V (2003) ThermoTRP channels and beyond: mechanisms of temperature sensation. Nat Rev Neurosci 4:529–539
Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A (2002) A TRP channel that senses cold stimuli and menthol. Cell 108:705–715
Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, Story GM, Colley S, Hogenesch JB, McIntyre P, Bevan S, Patapoutian A (2002) A heat-sensitive TRP channel expressed in keratinocytes. Science 296:2046–2049
Ramsey IS, Delling M, Clapham DE (2006) An introduction to TRP channels. Annu Rev Physiol 68:619–647
Roper SD (2006) Cell communication in taste buds. Cell Mol Life Sci 63:1494–1500
Souslova V, Cesare P, Ding Y, Akopian AN, Stanfa L, Suzuki R, Carpenter K, Dickenson A, Boyce S, Hill R, Nebenuis-Oosthuizen D, Smith AJ, Kidd EJ, Wood JN (2000) Warm-coding deficits and aberrant inflammatory pain in mice lacking P2X3 receptors. Nature 407:1015–1017
Stout CE, Costantin JL, Naus CC, Charles AC (2002) Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem 277:10482–10488
Talavera K, Nilius B, Voets T (2008) Neuronal TRP channels: thermometers, pathfinders and life-savers. Trends Neurosci 31:287–295
Togashi K, Hara Y, Tominaga T, Higashi T, Konishi Y, Mori Y, Tominaga M (2006) TRPM2 activation by cyclic ADP-ribose at body temperature is involved in insulin secretion. Embo J 25:1804–1815
Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, Vriens J, Cairns W, Wissenbach U, Prenen J, Flockerzi V, Droogmans G, Benham CD, Nilius B (2002) Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem 277:13569–13577
Wieraszko A, Goldsmith G, Seyfried TN (1989) Stimulation-dependent release of adenosine triphosphate from hippocampal slices. Brain Res 485:244–250
Xu H, Blair NT, Clapham DE (2005) Camphor activates and strongly desensitizes the transient receptor potential vanilloid subtype 1 channel in a vanilloid-independent mechanism. J Neurosci 25:8924–8937
Acknowledgements
S.M. is a JSPS Research Fellow. This work was supported by grants from the Japan Society for the Promotion of Science, Ministry of Education, Culture, Sport, Science, and Technology in Japan to M. T., K. M. and T. F.-T., and The Nakatomi Foundation to M. T.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
Characterization of mouse DRG neurons in response to heat (∼45°C) by Ca2+-imaging. Representative traces showing that heat application (black traces), evoked a rise in intracellular calcium levels (red traces) in DRG neurons obtained from wild-type (a), but not TRPV1-deficient (TRPV1KO, b) mice. 1 µM ionomycin (Ion) was applied to confirm cell viability (b). ATP (100 mM) and capsaicin (Cap, 1 µM) were also applied. Histogram (c) showing peak responses to heat as F − F0 (F peak − F initial) in DRG neurons. All the Cap-sensitive DRG neurons from wild-type mice responded to heat (a red trace in a and a red column in c) (0.74 ± 0.03, n = 190). None of the DRG neurons from TRPV1KO mice responded to 1 µM Cap or heat (a red trace in b and a green column in c; 0.08 ± 0.01, n = 220). Responses to ATP (100 µM) confirmed purinergic receptor activation (a and b) (PDF 70 kb)
Supplementary Figure 2
Heating up to 40°C did not cause death of keratinocytes or DRG neurons. a, b Phase-contrast images (left) and dead cells (propidium iodide, PI, positive cells shown by arrow heads, right) in the co-culture of wild-type keratinocytes with TRPV1-deficient DRG neurons at control (a) and heating (b). c Numbers of PI-positive dead cells were not different between control and heated cells (67.4 ± 15.9% and 65.8 ± 17.1%, respectively, n = 12) (PDF 64 kb)
Supplementary Figure 3
Dose–response curve for ATP-induced P2X2-mediated current responses, and the percentage of keratinocytes responding to heat stimulus. a A dose–response curve for ATP (1, 3, 10, and 30 µM)-induced P2X2-mediated current responses measured in HEK293 cells transfected with P2X2 cDNA. Current densities were normalized to the values evoked by 100 µM ATP. Data were fitted with a Hill equation with EC50 of 7.9 mM and a Hill co-efficient of 1.4. Parentheses indicate cell numbers tested. b Histograms showing percentage of keratinocytes from wild-type (WT), TRPV1-deficient (V1KO), TRPV4-deficient (V4KO), or TRPV3-deficient (V3KO) mice responding to heat stimuli revealed by Ca2+-imaging experiments; 76.4 ± 2.7% (538/708), 79.1 ± 2.9% (507/648), 77.3 ± 5.4% (567/711), and 26.6 ± 4.4% (179/640) of keratinocytes from WT, V1KO, V4KO, and V3KO (a red column) mice responded to heat stimulus (∼40°C). **p < 0.005, student t test (PDF 60 kb)
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Mandadi, S., Sokabe, T., Shibasaki, K. et al. TRPV3 in keratinocytes transmits temperature information to sensory neurons via ATP. Pflugers Arch - Eur J Physiol 458, 1093–1102 (2009). https://doi.org/10.1007/s00424-009-0703-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-009-0703-x