Abstract
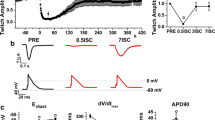
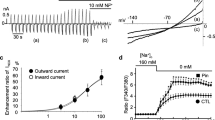
Intracellular Na+ concentration ([Na+]i) rises in the heart during ischemia, and on reperfusion, there is a transient rise followed by a return toward control. These changes in [Na+]i contribute to ischemic and reperfusion damage through their effects on Ca2+ overload. Part of the rise of [Na+]i during ischemia may be caused by increased activity of the cardiac Na+/H+ exchanger (NHE1), activated by the ischemic rise in [H+]i. In support of this view, NHE1 inhibitors reduce the [Na+]i rise during ischemia. Another possibility is that the rise of [Na+]i during ischemia is caused by Na+ influx through channels. We have reexamined these issues by use of two different NHE1 inhibitors, amiloride, and zoniporide, in addition to tetrodotoxin (TTX), which blocks voltage-sensitive Na+ channels. All three drugs produced cardioprotection after ischemia, but amiloride (100 μM) and TTX (300 nM) prevented the rise in [Na+]i during ischemia, whereas zoniporide (100 nM) did not. Both amiloride and zoniporide prevented the rise of [Na+]i on reperfusion, whereas TTX was without effect. In an attempt to explain these differences, we measured the ability of the three drugs to block Na+ currents. At the concentrations used, TTX reduced the transient Na+ current (I Na) by 11 ± 2% while amiloride and zoniporide were without effect. In contrast, TTX largely eliminated the persistent Na+ current (I Na,P) and amiloride was equally effective, whereas zoniporide had a substantially smaller effect reducing I Na,P to 41 ± 8%. These results suggest that part of the effect of NHE1 inhibitors on the [Na+]i during ischemia is by blockade of I Na,P. The fact that a low concentration of TTX eliminated the rise of [Na+]i during ischemia suggests that I Na,P is a major source of Na+ influx in this model of ischemia.



Similar content being viewed by others
References
Allen DG, Orchard CH (1987) Myocardial contractile function during ischemia and hypoxia. Circ Res 60:153–168
Allen DG, Xiao XH (2001) Letter to the Editor; Na+ entry during ischemia, reperfusion and preconditioning. Cardiovasc Res 50:164–166
Avkiran M (1999) Rational basis for use of sodium–hydrogen exchange inhibitors in myocardial ischemia. Am J Cardiol 83:10G–17G
Avkiran M (2001) Protection of the ischaemic myocardium by Na+/H+ exchange inhibitors: potential mechanisms of action. Basic Res Cardiol 96:306–311
Avkiran M, Gross G, Karmazyn M, Klein M, Murphy E, Ytrehus K (2001) Letter to the Editor—Na+/H+ exchange in ischemia, reperfusion and preconditioning. Cardiovasc Res 50:162–163
Avkiran M, Marber MS (2002) Na+/H+ exchange inhibitors for cardioprotective therapy: progress, problems and prospects. J Am Coll Cardiol 39:747–753
Carmeliet E (1999) Cardiac ionic currents and acute ischemia: from channels to arrhythmias. Physiol Rev 79:917–1017
Chattou S, Coulombe A, Diacono J, Le Grand B, John G, Feuvray D (2000) Slowly inactivating component of sodium current in ventricular myocytes is decreased by diabetes and partially inhibited by known Na+–H+ exchange blockers. J Mol Cell Cardiol 32:1181–1192
Cross HR, Radda GK, Clarke K (1995) The role of Na+/K+ ATPase activity during low flow ischemia in preventing myocardial injury: a 31P, 23Na and 87Rb NMR spectroscopic study. Magn Reson Med 34:673–685
Decking UK, Hartmann M, Rose H, Bruckner R, Meil J, Schrader J (1998) Cardioprotective actions of KC 12291. I. Inhibition of voltage-gated Na+ channels in ischemia delays myocardial Na+ overload. Naunyn Schmiedebergs Arch Pharmacol 358:547–553
Demaurex N, Romanek RR, Orlowski J, Grinstein S (1997) ATP dependence of Na+/H+ exchange. Nucleotide specificity and assessment of the role of phospholipids. J Gen Physiol 109:117–128
Frelin C, Vigne P, Lazdunski M (1984) The role of the Na+/H+ exchange system in cardiac cells in relation to the control of the internal Na+ concentration. A molecular basis for the antagonistic effect of ouabain and amiloride on the heart. J Biol Chem 259:8880–8885
Goss GG, Woodside M, Wakabayashi S, Pouyssegur J, Waddell T, Downey GP et al (1994) ATP dependence of NHE-1, the ubiquitous isoform of the Na+/H+ antiporter. Analysis of phosphorylation and subcellular localization. J Biol Chem 269:8741–8748
Haigney MC, Lakatta EG, Stern MD, Silverman HS (1994) Sodium channel blockade reduces hypoxic sodium loading and sodium-dependent calcium loading. Circulation 90:391–399
Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ (1981) Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391:85–100
Hammarstrom AK, Gage PW (2002) Hypoxia and persistent sodium current. Eur Biophys J 31:323–330
Hartmann M, Decking UK (1999) Blocking Na+–H+ exchange by cariporide reduces Na+-overload in ischemia and is cardioprotective. J Mol Cell Cardiol 31:1985–1995
Hartmann M, Decking UK (2003) R 56865 exerts cardioprotective properties independent of the intracellular Na(+)-overload in the guinea pig heart. Naunyn Schmiedebergs Arch Pharmacol 368:160–165
Hove MT, Jansen MA, Nederhoff MG, Van Echteld CJ (2007) Combined blockade of the Na+ channel and the Na+/H+ exchanger virtually prevents ischemic Na+ overload in rat hearts. Mol Cell Biochem (in press)
Jansen MA, Van Emous JG, Nederhoff MG, Van Echteld CJ (2004) Assessment of myocardial viability by intracellular 23Na magnetic resonance imaging. Circulation 110:3457–3464
Ju YK, Saint DA, Gage PW (1996) Hypoxia increases persistent sodium current in rat ventricular myocytes. J Physiol 497:337–347
Karmazyn M (1988) Amiloride enhances postischemic ventricular recovery: possible role of Na+–H+ exchange. Am J Physiol 255:H608–H615
Karmazyn M, Gan XT, Humphreys RA, Yoshida H, Kusumoto K (1999) The myocardial Na+–H+ exchange : structure, regulation, and its role in heart disease. Circ Res 85:777–786
Kleyman TR, Cragoe EJ (1988) Amiloride and its analogs as tools in the study of ion transport. J Membr Biol 105:1–21
Knight DR, Smith AH, Flynn DM, MacAndrew JT, Ellery SS, Kong JX et al (2001) A novel sodium–hydrogen exchanger isoform-1 inhibitor, zoniporide, reduces ischemic myocardial injury in vitro and in vivo. J Pharmacol Exp Ther 297:254–259
Le Grand B, Vie B, Talmant JM, Coraboeuf E, John GW (1995) Alleviation of contractile dysfunction in ischemic hearts by slowly inactivating Na+ current blockers. Am J Physiol 269:H533–H540
Marala RB, Brown JA, Kong JX, Tracey WR, Knight DR, Wester RT et al (2002) Zoniporide: a potent and highly selective inhibitor of human Na(+)/H(+) exchanger–1. Eur J Pharmacol 451:37–41
Meng HP, Lonsberry BB, Pierce GN (1991) Influence of perfusate pH on the postischemic recovery of cardiac contractile function: involvement of sodium–hydrogen exchange. J Pharmacol Exp Ther 258:772–777
Murphy E, Perlman M, London RE, Steenbergen C (1991) Amiloride delays the ischemia-induced rise in cytosolic free calcium. Circ Res 68:1250–1258
Park CO, Xiao XH, Allen DG (1999) Changes in intracellular sodium and pH in the rat heart during ischaemia: role of the Na+/H+ exchanger. Am J Physiol 276:H1581–H1590
Pike MM, Luo CS, Clark MD, Kirk KA, Kitakaze M, Madden MC et al (1993) NMR measurements of Na+ and cellular energy in ischemic rat heart: role of Na+/H+ exchange. Am J Physiol 265:H2017–H2026
Saint DA, Ju YK, Gage PW (1992) A persistent sodium current in rat ventricular myocytes. J Physiol 453:219–231
Takeo S, Tanonaka K (2004) Na+ overload-induced mitochondrial damage in the ischemic heart. Can J Physiol Pharmacol 82:1033–1043
Tani M (1990) Mechanisms of Ca2+ overload in reperfused ischemic myocardium. Ann Rev Physiol 52:543–559
Tani M, Neely JR (1989) Role of intracellular Na+ in Ca2+ overload and depressed recovery of ventricular function of reperfused ischemic rat hearts. Possible involvement of H+–Na+ and Na+–Ca2+ exchange. Circ Res 65:1045–1056
Theroux P, Chaitman BR, Danchin N, Erhardt L, Meinertz T, Schroeder JS et al (2000) Inhibition of the sodium–hydrogen exchanger with cariporide to prevent myocardial infarction in high-risk ischemic situations. Main results of the GUARDIAN trial. Guard during ischemia against necrosis (GUARDIAN) Investigators. Circulation 102:3032–3038
Turvey SE, Allen DG (1994) Changes in myoplasmic sodium concentration during exposure to lactate in perfused rat heart. Cardiovasc Res 28:987–993
Undrovinas AI, Fleidervish IA, Makielski JC (1992) Inward sodium current at resting potentials in single cardiac myocytes induced by the ischemic metabolite lysophosphatidylcholine. Circ Res 71:1231–1241
Van Emous J, Nederhoff MG, Ruigrok TJ, Van Echteld C (1997) The role of the Na+ channel in the accumulation of intracellular Na+ during myocardial ischemia: consequences for post-ischemic recovery. J Mol Cell Cardiol 29:85–96
Vandenberg JI, Metcalfe JC, Grace AA (1993) Mechanisms of pHi recovery after global ischemia in the perfused heart. Circ Res 72:993–1003
Verdonck F, Bielen FV, Ver-Donck L (1991) Preferential block of the veratridine-induced, non-inactivating Na+ current by R56865 in single cardiac Purkinje cells. Eur J Pharmacol 203:371–378
Wakabayashi S, Shigekawa M, Pouyssegur J (1997) Molecular physiology of vertebrate Na+/H+ exchangers. Physiol Rev 77:51–74
Weissberg PL, Little PJ, Cragoe EJJ, Bobik A (1989) The pH of spontaneously beating cultured rat heart cells is regulated by an ATP–calmodulin-dependent Na+/H+ antiport. Circ Res 64:676–685
Wu ML, Vaughan-Jones RD (1994) Effect of metabolic inhibitors and second messengers upon Na+–H+ exchange in the sheep cardiac Purkinje fibre. J Physiol 478:301–313
Xiao XH, Allen DG (1999) Role of the Na+/H+ exchanger during ischemia and preconditioning in the isolated rat heart. Circ Res 85:723–730
Xiao XH, Allen DG (2002) The role of endogenous angiotensin II in ischemia, reperfusion and preconditioning of the isolated rat heart. Pflügers Arch 445:643–650
Acknowledgment
This study is supported by the Australian National Health and Medical Research Council. IW was the recipient of a Northcote Graduate Scholarship, UK.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Williams, I.A., Xiao, Xh., Ju, Yk. et al. The rise of [Na+]i during ischemia and reperfusion in the rat heart—underlying mechanisms. Pflugers Arch - Eur J Physiol 454, 903–912 (2007). https://doi.org/10.1007/s00424-007-0241-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-007-0241-3