Abstract
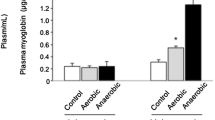
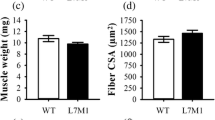
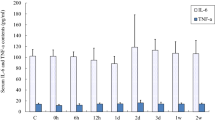
In the present study, we determined the impact of 5 and 10 days of muscle deconditioning induced by hindlimb suspension (HS) on the ubiquitin–proteasome system of protein degradation and caspase enzyme activities in rat soleus muscles. A second goal was to determine whether activities of matrix metalloproteinase-2/9 (MMP-2/9) and urokinase-type/tissue-type plasminogen activator (PAs) were responsive to HS. As expected, HS led to a pronounced atrophy of soleus muscle. Level of ubiquitinated proteins, chymotrypsin-like activity of 20S proteasome, and Bcl-2-associated gene product-1 protein level were all transitory increased in response to 5 days of HS. These changes may thus potentially account for the decrease in muscle mass observed in response to 5 days of HS. Caspase-3 activity was significantly increased throughout the experimental period, whereas activities of caspase-6, another effector caspase, and caspase-9, the mitochondrial-dependent activator of both caspase-3 and -6, were only increased in response to 10 days of HS. This suggests that caspase-3 may be regulated through mitochondrial-independent and mitochondrial-dependent mechanisms in response to HS. Finally, MMP-2/9 activities remained unchanged, whereas PAs activities were increased after 5 days of HS. Overall, these data suggest that time-dependent regulation of intracellular and extracellular proteinases are important in setting the new phenotype of rat soleus muscle in response to HS.






Similar content being viewed by others
References
Ahtikoski AM, Koskinen SO, Virtanen P, Kovanen V, Risteli J, Takala TE (2003) Synthesis and degradation of type IV collagen in rat skeletal muscle during immobilization in shortened and lengthened positions. Acta Physiol Scand 177:473–481
Allen DL, Linderman JK, Roy RR, Bigbee AJ, Grindeland RE, Mukku V, Edgerton VR (1997) Apoptosis: a mechanism contributing to remodeling of skeletal muscle in response to hindlimb unweighting. Am J Physiol 273:C579–C587
Attaix D, Ventadour S, Codran A, Bechet D, Taillandier D, Combaret L (2005) The ubiquitin–proteasome system and skeletal muscle wasting. Essays Biochem 41:173–186
Barani AE, Durieux AC, Sabido O, Freyssenet D (2003) Age-related changes in the mitotic and metabolic characteristics of muscle-derived cells. J Appl Physiol 95:2089–2098
Barani AE, Sabido O, Freyssenet D (2003) Mitotic activity of rat muscle satellite cells in response to serum stimulation: relation with cellular metabolism. Exp Cell Res 283:196–205
Bimston D, Song J, Winchester D, Takayama S, Reed JC, Morimoto RI (1998) BAG-1, a negative regulator of Hsp70 chaperone activity, uncouples nucleotide hydrolysis from substrate release. EMBO J 17:6871–6878
Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ (2001) Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294:1704–1708
Bonavaud S, Charriere-Bertrand C, Rey C, Leibovitch MP, Pedersen N, Frisdal E, Planus E, Blasi F, Gherardi R, Barlovatz-Meimon G (1997) Evidence of a non-conventional role for the urokinase tripartite complex (uPAR/uPA/PAI-1) in myogenic cell fusion. J Cell Sci 110:1083–1089
Caiozzo VJ, Baker MJ, Baldwin KM (1998) Novel transitions in MHC isoforms: separate and combined effects of thyroid hormone and mechanical unloading. J Appl Physiol 85:2237–2248
Carmeli E, Moas M, Reznick AZ, Coleman R (2004) Matrix metalloproteinases and skeletal muscle: a brief review. Muscle Nerve 29:191–197
Desplanches D, Ecochard L, Sempore B, Mayet-Sornay MH, Favier R (2004) Skeletal muscle HSP72 response to mechanical unloading: influence of endurance training. Acta Physiol Scand 180:387–394
Desplanches D, Mayet MH, Sempore B, Flandrois R (1987) Structural and functional responses to prolonged hindlimb suspension in rat muscle. J Appl Physiol 63:558–563
Du J, Wang X, Miereles C, Bailey JL, Debigare R, Zheng B, Price SR, Mitch WE (2004) Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J Clin Invest 113:115–123
Duguez S, Bihan MC, Gouttefangeas D, Feasson L, Freyssenet D (2003) Myogenic and nonmyogenic cells differentially express proteinases, Hsc/Hsp70, and BAG-1 during skeletal muscle regeneration. Am J Physiol Endocrinol Metab 285:E206–E215
Dupont-Versteegden EE, Fluckey JD, Knox M, Gaddy D, Peterson CA (2006) Effect of flywheel-based resistance exercise on processes contributing to muscle atrophy during unloading in adult rats. J Appl Physiol 101:202–212
Farout L, Lamare MC, Cardozo C, Harrisson M, Briand Y, Briand M (2000) Distribution of proteasomes and of the five proteolytic activities in rat tissues. Arch Biochem Biophys 374:207–212
Freyssenet D, Connor MK, Takahashi M, Hood DA (1999) Cytochrome c transcriptional activation and mRNA stability during contractile activity in skeletal muscle. Am J Physiol 277:E26–E32
Hoppeler H (1986) Exercise-induced ultrastructural changes in skeletal muscle. Int J Sports Med 7:187–204
Ikemoto M, Nikawa T, Takeda S, Watanabe C, Kitano T, Baldwin KM, Izumi R, Nonaka I, Towatari T, Teshima S, Rokutan K, Kishi K (2001) Space shuttle flight (STS-90) enhances degradation of rat myosin heavy chain in association with activation of ubiquitin–proteasome pathway. FASEB J 15:1279–1281
Irigoyen JP, Munoz-Canoves P, Montero L, Koziczak M, Nagamine Y (1999) The plasminogen activator system: biology and regulation. Cell Mol Life Sci 56:104–132
Kherif S, Dehaupas M, Lafuma C, Fardeau M, Alameddine HS (1998) Matrix metalloproteinases MMP-2 and MMP-9 in denervated muscle and injured nerve. Neuropathol Appl Neurobiol 24:309–319
Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, Price SR, Mitch WE, Goldberg AL (2004) Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J 18:39–51
Lee SW, Dai G, Hu Z, Wang X, Du J, Mitch WE (2004) Regulation of muscle protein degradation: coordinated control of apoptotic and ubiquitin–proteasome systems by phosphatidylinositol 3 kinase. J Am Soc Nephrol 15:1537–1545
Leeuwenburgh C, Gurley CM, Strotman BA, Dupont-Versteegden EE (2005) Age-related differences in apoptosis with disuse atrophy in soleus muscle. Am J Physiol Regul Integr Comp Physiol 288:R1288–R1296
Luders J, Demand J, Hohfeld J (2000) The ubiquitin-related BAG-1 provides a link between the molecular chaperones Hsc70/Hsp70 and the proteasome. J Biol Chem 275:4613–4617
Mayer MP, Bukau B (2005) Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci 62:670–684
Miller TA, Lesniewski LA, Muller-Delp JM, Majors AK, Scalise D, Delp MD (2001) Hindlimb unloading induces a collagen isoform shift in the soleus muscle of the rat. Am J Physiol Regul Integr Comp Physiol 281:R1710–R1717
Morey ER (1979) Spaceflight and bone turnover: correlation with a new rat model of weightlessness. BioScience 29:168–172
Nollen EA, Brunsting JF, Song J, Kampinga HH, Morimoto RI (2000) Bag1 functions in vivo as a negative regulator of Hsp70 chaperone activity. Mol Cell Biol 20:1083–1088
Oishi Y, Ishihara A, Talmadge RJ, Ohira Y, Taniguchi K, Matsumoto H, Roy RR, Edgerton VR (2001) Expression of heat shock protein 72 in atrophied rat skeletal muscles. Acta Physiol Scand 172:123–130
Oishi Y, Ishihara A, Yamamoto H, Miyamoto E (1998) Hindlimb suspension induces the expression of multiple myosin heavy chain isoforms in single fibres of the rat soleus muscle. Acta Physiol Scand 162:127–134
Reid MB (2005) Response of the ubiquitin–proteasome pathway to changes in muscle activity. Am J Physiol Regul Integr Comp Physiol 288:R1423–R1431
Reznick AZ, Menashe O, Bar-Shai M, Coleman R, Carmeli E (2003) Expression of matrix metalloproteinases, inhibitor, and acid phosphatase in muscles of immobilized hindlimbs of rats. Muscle Nerve 27:51–59
Siu PM, Pistilli EE, Alway SE (2005) Apoptotic responses to hindlimb suspension in gastrocnemius muscles from young adult and aged rats. Am J Physiol Regul Integr Comp Physiol 289:R1015–R1026
Stevens L, Sultan KR, Peuker H, Gohlsch B, Mounier Y, Pette D (1999) Time-dependent changes in myosin heavy chain mRNA and protein isoforms in unloaded soleus muscle of rat. Am J Physiol 277:C1044–C1049
Stevenson EJ, Giresi PG, Koncarevic A, Kandarian SC (2003) Global analysis of gene expression patterns during disuse atrophy in rat skeletal muscle. J Physiol 551:33–48
Taillandier D, Aurousseau E, Meynial-Denis D, Bechet D, Ferrara M, Cottin P, Ducastaing A, Bigard X, Guezennec CY, Schmid HP et al (1996) Coordinate activation of lysosomal, Ca2+-activated and ATP–ubiquitin-dependent proteinases in the unweighted rat soleus muscle. Biochem J 316(Pt 1):65–72
Taillandier D, Combaret L, Pouch MN, Samuels SE, Bechet D, Attaix D (2004) The role of ubiquitin–proteasome-dependent proteolysis in the remodelling of skeletal muscle. Proc Nutr Soc 63:357–361
Thomason DB, Herrick RE, Surdyka D, Baldwin KM (1987) Time course of soleus muscle myosin expression during hindlimb suspension and recovery. J Appl Physiol 63:130–137
Vermaelen M, Marini JF, Chopard A, Benyamin Y, Mercier J, Astier C (2005) Ubiquitin targeting of rat muscle proteins during short periods of unloading. Acta Physiol Scand 185:33–40
Wittwer M, Fluck M, Hoppeler H, Muller S, Desplanches D, Billeter R (2002) Prolonged unloading of rat soleus muscle causes distinct adaptations of the gene profile. FASEB J 16:884–886
WMA (2002) Guiding principles for research involving animals and human beings. Am J Physiol Regul Integr Comp Physiol 283:R281–R283
Acknowledgements
The authors thank D. Desplanches for critical review of the manuscript. We are grateful to F. Sabot for his help with enzyme assays.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berthon, P., Duguez, S., Favier, F.B. et al. Regulation of ubiquitin–proteasome system, caspase enzyme activities, and extracellular proteinases in rat soleus muscle in response to unloading. Pflugers Arch - Eur J Physiol 454, 625–633 (2007). https://doi.org/10.1007/s00424-007-0230-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-007-0230-6