Abstract.
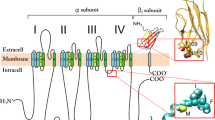
Voltage-gated Na channels comprise four homologous domains each consisting of six transmembrane segments (S1–S6) linked by loops. The linkers between segments S5 and S6 in each domain (P-loops), denoted as SS1-SS2, form the pore of the channel. It is believed that the SS1 region of the P-loops dips into, while the SS2 region exits out of the membrane. We have reported previously that residues A728 and D730 (in SS1 of domain II) contribute to the external vestibule of the pore of the rat skeletal muscle Na channel (Nav1.4). In this study, we examined the role of a conserved neighbouring tryptophan residue at position 736 (W736) in the pore formation. The W736 residue of Nav1.4 was replaced by a cysteine using site-directed mutagenesis. Complementary RNAs encoding the wild-type and mutant channels were injected into Xenopus laevis oocytes and macroscopic Na+ currents measured using the two-microelectrode voltage-clamp technique. The W736C mutant showed increased channel sensitivity to externally applied Cd2+ and methanethiosulphonate-ethyltrimethylammonium (MTSET). Furthermore, micromolar concentrations of Cd2+ reduced single-channel current amplitude in the Nav1.4/W736C mutant without affecting its voltage dependence. However, only small differences in tetrodotoxin and µ-conotoxin GIIIA affinity were observed between the wild-type and mutant channels. Replacing Na+ with other cations – K+, Li+, Cs+ or NH4 + – did not change the ion permeation sequence of the Nav1.4/W736C mutant channel. The results suggest that W736 contributes to the formation of the pore, close to the mouth of the channel, but is not part of the selectivity filter.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Carbonneau, E., Vijayaragavan, K. & Chahine, M. A tryptophan residue (W736) in the amino-terminus of the P-segment of domain II is involved in pore formation in Nav1.4 voltage-gated sodium channels. Pflugers Arch - Eur J Physiol 445, 18–24 (2002). https://doi.org/10.1007/s00424-002-0887-9
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00424-002-0887-9