Abstract.
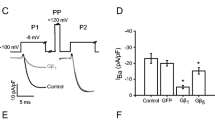
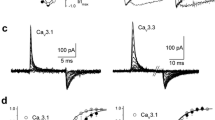
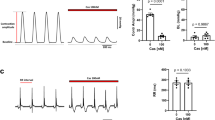
Modulation of calcium channels by both auxiliary subunits and G proteins was studied in cell-attached patches from COS-7 cells transfected with Cav2.2 channel subunits (N-type, α1B and either β1b or β2a). These were co-expressed with either Gβ1γ2 or the Gβγ-binding domain of β-adrenergic-receptor kinase-1 to sequester endogenous Gβγ. Since G protein modulation of Cav channels may affect both inactivation and activation, we examined Gβγ modulation of Cav2.2 channels in the presence of two different β-subunits that affect inactivation differently and compared in detail the single-channel characteristics of N-type channels expressed with either of these β-subunit isoforms. The single-channel mean amplitude and mean open time were not influenced by the transfection combination. However, the mean closed time at +40 mV was increased for both β1b and β2a-subunits by co-transfection with Gβ1γ2. This effect was absent at lower voltages as examined for channels with the β1b-subunit. The distribution of latency-to-first-opening of Cav2.2 channels was similar for both β-subunit isoforms. However, the inclusion of the β2a subunit resulted in channels with an additional, prominent, slow activation phase. Co-transfection of Gβ1γ2 with Cav2.2 channels markedly reduced the ensemble current amplitude and slowed the first latency. The inhibition imposed by Gβ1γ2 was largely independent of the β-subunit species. Facilitation of Gβγ-modulated currents (the channel response following a large and brief depolarising prepulse) was observed for channels with both β-subunits and involved mainly enhancement of the activation, as assessed by the faster first latency. The inactivation process was strongly dependent on the β-subunit species, with β1b supporting inactivation and β2a reducing this process. This difference was assessed by estimation of both steady-state inactivation (prepulse influence on test pulse responses) and the inactivation time course during depolarisation. At +40 mV, channels with the β1b-subunit had a fast component of inactivation (time constant ~180 ms, 50%) and a slow phase with time constant of ~1 s, while the β2a-subunit supported only a very slow inactivation process with time constant of ~5 s. Co-transfection of Gβ1γ2 with the Cav2.2 channel had no effect on the inactivation properties with either β-subunit. In summary, we show that the inactivation properties of expressed Cav2.2 channels depend largely on the β-subunit species and to a minor extent only on the presence or absence of the Gβγ modulator. Furthermore, the activation, amplitude, mean open and closed times and G protein modulation of N-type channels were similar for both β1b- and β2a-subunits.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Meir, A., Dolphin, A.C. Kinetics and Gβγ modulation of Cav2.2 channels with different auxiliary β subunits. Pflügers Arch - Eur J Physiol 444, 263–275 (2002). https://doi.org/10.1007/s00424-002-0803-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-002-0803-3