Abstract
Purpose
The effect of hepatic steatosis on the development of colorectal liver metastases (CRLM) remains unknown. This study evaluated the usefulness of fat signal fraction assessed with magnetic resonance imaging (MRI) and the effect of hepatic steatosis on hepatic recurrences following initial hepatectomy for CRLM.
Methods
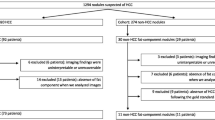
Between January 2013 and December 2019, 64 patients underwent initial hepatectomy for CRLM. The medical records of these patients were reviewed to evaluate the recurrence and survival outcomes.
Results
The fat signal fraction was positively correlated with the nonalcoholic fatty liver disease activity score and liver-spleen ratio. Recurrence following the initial hepatectomy was observed in 48/64 patients, and hepatic recurrence was observed in 30/64 patients. The fat signal fraction was significantly higher in patients with hepatic recurrence after initial hepatectomy. The hepatic recurrence rate was 69.2% in patients with fat signal fraction ≥ 0.0258, which was significantly higher than that in patients with fat signal fraction < 0.0258. Hepatic recurrence-free survival rate was significantly higher in patients with fat signal fraction < 0.0258 than in those with fat signal fraction ≥ 0.0258. Multivariate analyses revealed that fat signal fraction ≥ 0.0258 was an independent risk factor for hepatic recurrence.
Conclusion
The fat signal fraction assessed with MRI was significantly associated with hepatic recurrence following initial hepatectomy for CRLM.






Similar content being viewed by others
Data availability
The data that support the findings of the present study are available from the corresponding author upon reasonable request.
References
Bray F et al (2018) Global, cancer statistics GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. https://doi.org/10.3322/caac.21492
Manfredi S et al (2006) Epidemiology and management of liver metastases fromcolorectal cancer. Ann Surg 244:254–259. https://doi.org/10.1097/01.sla.0000217629.94941.cf
Hackl C et al (2014) Treatment of colorectal liver metastases in Germany: a ten-year population-based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer 14:810. https://doi.org/10.1186/1471-2407-14-810
Engstrand J, Nilsson H, Strömberg C, Jonas E, Freedman J (2018) Colorectal cancer liver metastases - a population-based study on incidence, management and survival. BMC Cancer 18:78. https://doi.org/10.1186/s12885-017-3925-x
Margonis GA, Kreis ME, Wang JJ, Kamphues C, Wolfgang CL, Weiss MJ (2020) Impact and clinical usefulness of genetic data in the surgical management of colorectal cancer liver metastasis: a narrative review. Hepatobiliary Surg Nutr 9:705–716. https://doi.org/10.21037/hbsn.2019.10.05
Paget S (1989) The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev 8:98–101
Weber CE, Kuo PC (2012) The tumor microenvironment. Surg Oncol 21:172–177. https://doi.org/10.1016/j.suronc.2011.09.001
Galindo-Pumarino C, Collado M, Herrera M, Pena C (2021) Tumor microenvironment in metastatic colorectal cancer: the arbitrator in patients’ outcome. Cancers (Basel) 13:1130. https://doi.org/10.3390/cancers13051130
Zhao J, van Mierlo KMC, Gomez-Ramirez J et al (2017) Systematic review of the influence of chemotherapy-associated liver injury on outcome after partial hepatectomy for colorectal liver metastases. Br J Surg 104:990–1002. https://doi.org/10.1002/bjs.10572
Paternostro R, Sieghart W, Trauner M, Pinter M (2021) Cancer and hepatic steatosis ESMO Open 6:100185. https://doi.org/10.1016/j.esmoop.2021.100185
Doherty DT, Coe PO, Rimmer L, Lapsia S, Krige A, Subar DA (2019) Hepatic steatosis in patients undergoing resection of colorectal liver metastases: a target for prehabilitation? A narrative review. Surg Oncol 30:147–158. https://doi.org/10.1016/j.suronc.2019.07.007
Fernandez FG, Ritter J, Goodwin JW, Linehan DC, Hawkins WG, Strasberg SM (2005) Effect of steatohepatitis associated with irinotecan or oxaliplatin pretreatment on resectability of hepatic colorectal metastases. J Am Coll Surg 200:845–853. https://doi.org/10.1016/j.jamcollsurg.2005.01.024
Li Y, Su X, Rohatgi N et al (2020) Hepatic lipids promote liver metastasis. JCI Insight 5:e136215. https://doi.org/10.1172/jci.insight.136215
Ramos E, Torras J, Llado L, Rafecas A, Serrano T, Lopez-Gordo S, Busquets J, Fabregat J (2016) The influence of steatosis on the short- and long-term results of resection of liver metastases from colorectal carcinoma. HPB (Oxford) 18:389–396. https://doi.org/10.1016/j.hpb.2015.12.002
Narayan RR, Harris JW, Chou JF et al (2020) Prediction of recurrence patterns from hepatic parenchymal disease after resection of colorectal liver metastases. Ann Surg Oncol 27:188–195. https://doi.org/10.1245/s10434-019-07934-3
Ma J (2008) Dixon techniques for water and fat imaging. J Magn Reson Imaging 28:543–558. https://doi.org/10.1002/jmri.21492
Reeder SB, Sirlin C (2010) Quantification of liver fat with magnetic resonance imaging. Magn Reson Imaging Clin N Am 18:337–357. https://doi.org/10.1016/j.mric.2010.08.013
Dixon WT (1984) Simple proton spectroscopic imaging. Radiology 153:189–194. https://doi.org/10.1148/radiology.153.1.6089263
Onishi H, Theisen D, Dietrich O, Reiser MF, Zech CJ (2014) Hepatic steatosis: effect on hepatocyte enhancement with gadoxetate disodium-enhanced liver MR imaging. J Magn Reson Imaging 39:42–50. https://doi.org/10.1002/jmri.24136
Kleiner DE, Brunt EM, Van Natta M et al (2005) Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41:1313–1321. https://doi.org/10.1002/hep.20701
Narayan RR, Harris JW, Chou JF et al (2019) Prediction of recurrence patterns from hepatic parenchymal disease after resection of colorectal liver metastases. Ann Surg Oncol 27:188–195. https://doi.org/10.1245/s10434-019-07934-3
Hu X, Marietta A, Dai WX, Li YQ, Ma XJ, Zhang L, Cai SJ, Peng JJ (2020) Prediction of hepatic metastasis and relapse in colorectal cancers based on concordance analyses with liver fibrosis scores. Clin Transl Med 9:13. https://doi.org/10.1186/s40169-020-0264-3
Kondo T, Okabayashi K, Hasegawa H, Tsuruta M, Shigeta K, Kitagawa Y (2016) The impact of hepatic fibrosis on the incidence of liver metastasis from colorectal cancer. Br J Cancer 115:34–39. https://doi.org/10.1038/bjc.2016.155
VanSaun MN, Lee IK, Washington MK, Matrisian L, Gorden DL (2009) High fat diet induced hepatic steatosis establishes a permissive microenvironment for colorectal metastases and promotes primary dysplasia in a murine model. Am J Pathol 175:355–364. https://doi.org/10.2353/ajpath.2009.080703
Karube H, Masuda H, Hayashi S, Ishii Y, Nemoto N (2000) Fatty liver suppressed the angiogenesis in liver metastatic lesions. Hepatogastroenterology 47:1541–1545
Masaki S, Hashimoto Y, Kunisho S, Kimoto A, Kitadai Y (2020) Fatty change of the liver microenvironment influences the metastatic potential of colorectal cancer. Int J Exp Pathol 101:162–170. https://doi.org/10.1111/iep.12371
Nordlinger B, Quilichini MA, Parc R, Hannoun L, Delva E, Huguet C (1987) Hepatic resection for colorectal liver metastases. Influence on survival of preoperative factors and surgery for recurrences in 80 patients. Ann Surg 205:256–263. https://doi.org/10.1097/00000658-198703000-00007
Ambiru S, Miyazaki M, Ito H, Nakagawa K, Shimizu H, Kato A, Nakamura S, Omoto H, Nakajima N (1998) Resection of hepatic and pulmonary metastases in patients with colorectal carcinoma. Cancer 82:274–278. https://doi.org/10.1002/(SICI)1097-0142(19980115)82:2%3c274::AID-CNCR5%3e3.0.CO;2-R
Chua TC, Saxena A, Liauw W, Chu F, Morris DL (2012) Hepatectomy and resection of concomitant extrahepatic disease for colorectal liver metastases–a systematic review. Eur J Cancer 48:1757–1765. https://doi.org/10.1016/j.ejca.2011.10.034
Wicherts DA, de Haas RJ, Salloum C, Andreani P, Pascal G, Sotirov D, Adam R, Castaing D, Azoulay D (2013) Repeat hepatectomy for recurrent colorectal metastases. Br J Surg 100:808–818. https://doi.org/10.1002/bjs.9088
Sakamaki Y, Ishida D, Tanaka R (2020) Prognosis of patients with recurrence after pulmonary metastasectomy for colorectal cancer. Gen Thorac Cardiovasc Surg 68:1172–1178. https://doi.org/10.1007/s11748-020-01368-5
Oba M, Hasegawa K, Shindoh J, Yamashita S, Sakamoto Y, Makuuchi M, Kokudo N (2016) Survival benefit of repeat resection of successive recurrences after the initial hepatic resection for colorectal liver metastases. Surgery 159:632–640. https://doi.org/10.1016/j.surg.2015.09.003
Oba M, Hasegawa K, Matsuyama Y, Shindoh J, Mise Y, Aoki T, Sakamoto Y, Sugawara Y, Makuuchi M, Kokudo N (2014) Discrepancy between recurrence-free survival and overall survival in patients with resectable colorectal liver metastases: a potential surrogate endpoint for time to surgical failure. Ann Surg Oncol 21:1817–1824. https://doi.org/10.1245/s10434-014-3504-1
Esterson YB, Grimaldi GM (2018) Radiologic imaging in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Clin Liver Dis 22:93–108. https://doi.org/10.1016/j.cld.2017.08.005
van Dijk DPJ, Krill M, Farshidfar F et al (2019) Host phenotype is associated with reduced survival independent of tumour biology in patients with colorectal liver metastases. J Cachexia Sarcopenia Muscle 10:123–130. https://doi.org/10.1002/jcsm.12358
Liu YW, Lu CC, Chang CD et al (2020) Prognostic value of sarcopenia in patients with colorectal liver metastases undergoing hepatic resection. Sci Rep 10:6459. https://doi.org/10.1038/s41598-020-63644-x
Kobayashi A, Kaido T, Hamaguchi Y, Okumura S, Shirai H, Kamo N, Yagi S, Taura K, Okajima H, Uemoto S (2018) Impact of visceral adiposity as well as sarcopenic factors on outcomes in patients undergoing liver resection for colorectal liver metastases. World J Surg 42:1180–1191. https://doi.org/10.1007/s00268-017-4255-5
Lodewick TM, van Nijnatten TJ, van Dam RM, van Mierlo K, Dello SA, Neumann UP, Olde Damink SW, Dejong CH (2015) Are sarcopenia, obesity and sarcopenic obesity predictive of outcome in patients with colorectal liver metastases? HPB (Oxford) 17:438–446. https://doi.org/10.1111/hpb.12373
Acknowledgements
We would like to thank Dr. Takuro Horikoshi, Department of Diagnostic Radiology and Radiation Oncology, Graduate School of Medicine, Chiba University, for the valuable advice regarding the MRI protocol.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The retrospective study was performed in accordance with the principles of the Declaration of Helsinki. The study was approved by the Institutional Ethics Committee of the College of Medicine, Chiba University.
Consent to participate
Because of the retrospective nature of the study, informed consent was waived by the Institutional Ethics Committee of the College of Medicine, Chiba University. As an alternative, the opt-out consent was approved by the committee and obtained via our websites, where permission was requested for the use of the participants’ personal information in this study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sakai, N., Hayano, K., Mishima, T. et al. Fat signal fraction assessed with MRI predicts hepatic recurrence following hepatic resection for colorectal liver metastases. Langenbecks Arch Surg 407, 1981–1989 (2022). https://doi.org/10.1007/s00423-022-02482-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-022-02482-z