Abstract
Purpose
Esophagectomy with gastric tube reconstruction is often complicated postoperatively by duodenogastric reflux and/or delayed gastric emptying and the accompanying symptoms, leading to patients being dissatisfied with their quality of life (QOL). Medical interventions to relieve patients of their symptoms are rarely effective. We began, in 2018, performing double tract-like gastric tube reconstruction, and, in a pilot study, we compared postoperative QOL between patients in whom this experimental reconstruction was performed and those in whom conventional reconstruction was performed.
Methods
Included in the study were 33 patients who underwent thoracoscopic McKeown esophagectomy with two- or three-field lymph node dissection for thoracic esophageal cancer between April 2015 and March 2020. A gastric tube about 4 cm in width was created in all patients, and in 14 of the patients (DT group), a double tract was appended by anastomosing the elevated jejunum to the anterior wall of the gastric tube, QOL was assessed 10–14 months later by means of the DAUGS-32 questionnaire, and bile reflux and the presence or absence of food residue were assessed by upper gastrointestinal endoscopy.
Results
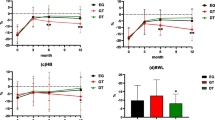
DAUGS-32 food passage dysfunction, nausea and vomiting, and reflux symptoms scores were significantly lower in the DT group than in the conventional reconstruction group. There was no significant between-group difference in the incidence of postoperative complications. No food residue was seen in DT patients’ gastric tube, and no reflux esophagitis was observed.
Conclusion
Double tract-like gastric tube reconstruction shows promise as an effective means of improving patients’ post-esophagectomy QOL.



Similar content being viewed by others
Data availability
The data used in this study includes the patient’s personal body and medical information. It is impossible to share the information because of the protection of the patient’s private information.
References
Watanabe M, Otake R, Kozuki R et al (2020) Recent progress in multidisciplinary treatment for patients with esophageal cancer. Surg Today 50:12–20
Akiyama H, Tsurumaru M, Udagawa H, Kajiyama Y (1994) Radical lymph node dissection for cancer of the thoracic esophagus. Ann Surg 220:364–373
Isono K, Sato H, Nakayama K (1991) Results of a nationwide study on the three-field lymph node dissection of esophageal cancer. Oncol 48:411–420
Elliott JA, Docherty NG, Eckhardt HG et al (2017) Weight loss, satiety, and the postprandial gut hormone response after esophagectomy: a prospective study. Ann Surg 266:82–90
Takeuchi H, Miyata H, Gotoh M et al (2014) A risk model for esophagectomy using data of 5354 patients included in a Japanese nationwide web-based database. Ann Surg 260:259–266
Harada K, Yoshida N, Baba Y et al (2017) Pyloroplasty may reduce weight loss 1 year after esophagectomy. Dis Esophagus 31:1–8
Aly A, Jamieson GG (2004) Reflux after oesophagectomy. Br J Surg 91:137–141
Shirahata K, Okabayashi T, Nagaoka K, Shiratori T (1971) Effect of vagotomy on the antral motor function. Nihon Heikatsukin Gakkai Zasshi 7:28–34
Collard JM, Romagnoli R, Otte JB, Kestens PJ (1998) The denervated stomach as an esophageal substitute is a contractile organ. Ann Surg 227:33–39
Yamagishi T, Debas HT (1978) Control of gastric emptying: interaction of the vagus and pyloric antrum. Ann Surg 187:91–94
Wilbur BG, Kelly KA (1973) Effect of proximal gastric, complete gastric, and truncal vagotomy of canine gastric electric activity, motility, and emptying. Ann Surg 178:295–303
Ukegiini K, Vetter D, Fehr R, Dirr V, Gubler C, Gutschow CA (2021) Functional syndromes and symptom-orientated aftercare after esophagectomy. Langenbecks Arch Surg 406:2249–2261
Ludwig DJ, Thirlby RC, Low DE (2001) A prospective evaluation of dietary status and symptoms after near-total esophagectomy without gastric emptying procedure. Am J Surg 181:454–458
Palmes D, Weilinghoff M, Colombo-Beckmann M, Senninger N, Bruewer M (2007) Effect of pyloric drainage procedures on gastric passage and bile reflux after esophagectomy with gastric conduit reconstruction. Langenbecks Arch Surg 392:135–141
Demos NJ, Kulkarni VA, Port A, Micale J (1993) Control of postresection gastroesophageal reflux: the intercostal pedicle esophagogastropexy experience of 26 years. Ann Surg 59:137–148
Ahn SH, Jung DH, Son SY, Lee CM, Park DJ, Kim HH (2014) Laparoscopic double-tract proximal gastrectomy for proximal early gastric cancer. Gastric Cancer 17:562–570
Kitagawa Y, Uno T, Oyama T et al (2019) Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society. Esophagus 16:1–24
Kitagawa Y, Uno T, Oyama T et al (2019) Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: part 2. Esophagus 16:25–43
Nakamura M, Kido Y, Yano M, Hosoya Y (2005) Reliability and validity of a new scale to assess postoperative dysfunction after resection of upper gastrointestinal carcinoma. Surg Today 35:535–542
Yano M, Sugimura K, Miyata H et al (2020) Randomized comparison of gastric tube reconstruction with and without duodenal diversion plus Roux-en-Y anastomosis after esophagectomy. Ann Surg 272:48–54
Armstrong D, Bennett JR, Blum AL et al (1996) The endoscopic assessment of esophagitis: a progress report on observe agreement. Gastroenterol 111:85–92
Fukuhara K, Osugi H, Lee S, Morimura K, Kishida S, Nishizawa S, Iwasaki H, Gyobu K (2009) Remnant gastritis should be evaluated histologically rather than endoscopically. Hepatogastroentrol 56:905–907
Konradsson M, van Berge Henegouwen MI, Bruns C et al (2020) Diagnostic criteria and symptom grading for delayed gastric conduit emptying after esophagectomy for cancer: international expert consensus based on a modified Delphi process. Dis Esophagus 33:1–9
Clavien PA, Barkun J, de Oliveira ML et al (2009) The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250:187–196
Standring S, Borley NR, Gray H (2008) Gray’s anatomy: the anatomical basis of clinical practice. Churchill Livingstone, London
Cheung HC, Siu KF, Wong J (1987) Is pyloroplasty necessary in esophageal replacement by stomach? A prospective, randomized controlled trial. Surgery 102:19–24
Gupta S, Chattopadhyay TK, Gopinath PG (1989) Emptying of the intrathoracic stomach with and without pyloroplasty. Am J Gastroenterol 84:921–923
Law S, Cheung MC, Fok M (1997) Pyloroplasty and pyloromyotomy in gastric replacement of the esophagus after esophagectomy: a randomized controlled trial. J Am Coll Surg 184:630–636
Arya S, Markar SR, Karthikesalingam A, Hanna GB (2015) The impact of pyloric drainage on clinical outcome following esophagectomy: a systematic review. Dis Esophagus 28:326–335
Ohwada S, Satoh Y, Kawate S et al (2001) Low-dose erythromycin reduces delayed gastric emptying and improves gastric motility after Billroth I pylorus-preserving pancreaticoduodenectomy. Ann Surg 234:668–674
Gutschow CA, Collard JM, Romagnoli R, Michel JM, Salizzoni M, Holscher AH (2001) Bile exposure of the denervated stomach as an esophageal substitute. Ann Thorac Surg 71:1786–1791
Dresner SM, Wayman J, Bennet MK, Hayes N, Griffin SM (2003) A human model of duodeno-gastro-esophageal reflux in the development of Barrett’s metaplasia. Br J Surg 90:1120–1128
Watkins D, Prevedel A, Harper F (1954) A method of preventing peptic esophagitis following esophagogastrostomy. J Thorac Surg 28:367–381
Wooler G (1956) Reconstruction of the cardia and fundus of the stomach. Thorax 11:275–280
Yalav E, Ercan S (2000) Reservoir and globe-type antireflux surgical techniques in intrathoracic esophagogastrostomies. Dis Esoph 13:282–287
Lortat-Jacob J, Maillard J, Fekete F (1960) A procedure to prevent reflux after esophagogastric resection: experience with 17 patients. Surgery 50:600–611
Yano M, Motoori M, Tanaka K et al (2012) Prevention of gastroduodenal content reflux and delayed gastric emptying after esophagectomy: gastric tube reconstruction with duodenal diversion plus Roux-en-Y anastomosis. Dis Esophagus 25:181–187
Arnold M, Laversanne M, Brown LM, Devesa SS, Bray F (2017) Predicting the future burden of esophageal cancer by histological subtype: international trends in incidence up to 2030. Am J Gastroenterol 112:1247–1255
Berry MF, Atkins BZ, Tong BC, Harpole DH, D’Amico TA, Onaitis MW (2010) A comprehensive evaluation for aspiration after esophagectomy reduces the incidence of postoperative pneumonia. J Thorac Cardiovasc Surg 140:1266–1271
Satake K, Nishiwaki H, Umeyama K (1985) Comparative studies of plasma secretin response after reconstructive surgery of the stomach and pancreas. Ann Surg 201:447–451
Okada G, Momoki C, Habu D et al (2019) Effect of postoperative oral intake on prognosis for esophageal cancer. Nutrients 11:1338
Zhang H, Shang X, Ren P et al (2019) The predictive value of a preoperative systemic immune-inflammation index and prognostic nutritional index in patients with esophageal squamous cell carcinoma. J Cell Physiol 234:1794–1802
Kalmar K, Németh J, Kelemen D, Agoston E, Horvath OP (2006) Postprandial gastrointestinal hormone production is different, depending on the type of reconstruction following total gastrectomy. Ann Surg 243:465–471
Nakajima K, Kawano M, Kinami S, Fujimura T, Miwa K, Tonami N (2005) Dual-radionuclide simultaneous gastric emptying and bile transit after gastric surgery with double-tract reconstruction. Ann Nucl Med 19:185–191
Author information
Authors and Affiliations
Contributions
Daisuke Fujimoto: conceptualization, methodology, writing—original draft, visualization. Keizo Taniguchi: conceptualization, investigation. Junpei Takashima: formal analysis, resources, data curation. Fumihiko Miura: formal analysis, resources, data curation. Hirotoshi Kobayashi: validation, Writing—review and editing, supervision, projection administration, funding acquisition.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Ethical approval was obtained in all participating centers.
Informed consent
Informed consent was obtained from all individual participants included in the study prior to recruitment.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 32767 KB)
Supplementary file2 (MP4 1154 KB)
Rights and permissions
About this article
Cite this article
Fujimoto, D., Taniguchi, K., Takashima, J. et al. Double tract-like gastric tube reconstruction decreases the incidences of delayed gastric emptying and bile reflux after esophagectomy: results of a pilot study of an experimental technique. Langenbecks Arch Surg 407, 1431–1439 (2022). https://doi.org/10.1007/s00423-022-02461-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-022-02461-4