Abstract
Introduction
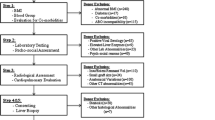
Potential live liver donors with non-alcoholic steatohepatitis (NASH) are rejected upfront for donation in live donor liver transplantation (LDLT). Herein, we share our experience of the feasibility of live liver donation in donors with NASH after successful donor optimization.
Materials and methods
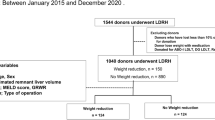
Prospectively collected data of 410 consecutive donor hepatectomies from June 2011 to January 2018 were analyzed.
Results
During the study period, NASH was diagnosed histopathologically in 17 donors. Four donors were rejected in view of grade 2 fibrosis on histology. Out of remaining 13 donors, six became eligible for donation following lifestyle changes, dietary modifications, and target weight reduction of ≥5%. Reversal of NASH was confirmed on repeat liver biopsy in all the 6 donors. Five out of 6 underwent right lobe (without MHV) donor hepatectomies, while one had left lobe donation. These donors had significantly higher peak bilirubin levels in the immediate post-operative period as compared to other non-NASH donors (4.00 ± 0.32 vs. 2.57 ± 1.77 mg/dL, p = 0.043). In addition, post-hepatectomy normalization of hyperbilirubinemia, if any, was slower in donors with NASH (7 ± 1.3 vs. 5 ± 1.7 days, p = 0.016). However, none of these donors had post-hepatectomy liver failure. All these donors were discharged after an average hospital stay of 8 ± 1.7 days. Their respective recipients had uneventful post-operative courses without complications. Both the recipients and donors are having satisfactory liver functions after 46.7 ± 10.2 months of follow-up.
Conclusion
Scrupulous selection of live liver donors with NASH can open a door for expanding the organ pool in LDLT after a successful donor optimization program.




Similar content being viewed by others
References
Pamecha V, Mahansaria SS, Bharathy KG, Kumar S, Sasturkar SV, Sinha PK et al (2016) Selection and outcome of the potential live liver donor. Hepatol Int 10(4):657–664
Fan ST, Lo CM, Liu CL, Yong BH, Chan JK, Ng IO (2000) Safety of donors in live donor liver transplantation using right lobe grafts. Arch Surg 135(3):336–340
Oshita A, Tashiro H, Amano H, Kobayashi T, Onoe T, Ide K et al (2012) Safety and feasibility of diet-treated donors with steatotic livers at the initial consultation for living-donor liver transplantation.Transplantation. 93(10):1024–1030
Choudhary NS, SarafN, Saigal S, Gautam D, Lipi L, RastogiA,et al. Rapid reversal of liver steatosis with lifestyle modification in highly motivated liver donors. J ClinExpHepatol 2015; 5(2):123–126
R. Agha, A. Abdall-Razak, E. Crossley, N. Dowlut, C. Iosifidis, G. Mathew, et al., STROCSS 2019 Guideline: strengthening the reporting of cohort studies in surgery, Int J Surg 72 (2019) 156–165
Marsman WA, Wiesner RH, Rodriguez L, Batts KP, Porayko MK, Hay JE et al (1996) Use of fatty donor liver is associated with diminished early patient and graft survival. Transplantation. 62(9):1246–1251
Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR (1999) Non-alcoholicsteatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 94(9):2467–2474
Pamecha V, Bharathy KG, Kumar S, Sasturkar SV, Sinha PK (2016) Biliary complications after living donor hepatectomy: a first report from India. Liver Transpl 22(5):607–614
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D et al (2005) The “50-50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg 242(6):824–828
Olthoff KM, Kulik L, Samstein B, Abecassis M, Emond J, ShakedA,et al. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl 2010; 16(8):943–949
Wong VW, Wong GL, Choi PC, Chan AW, Li MK, Chan HY et al (2010) Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut. 59(7):969–974
Anurag L, Aniket S, Shalik J, Amarja L, Dhananjay R, Sachin J (2015) Non-alcoholic fatty liver disease prevalence and associated risk factors–a study from rural sector of Maharashtra. Trop Gastroenterol 36(1):25–30
Majumdar A, Misra P, Sharma S, Kant S, Krishnan A, Pandav CS (2016) Prevalence of nonalcoholic fatty liver disease in an adult population in a rural community of Haryana. India Indian J Public Health 60(1):26–33
Das K, Das K, Mukherjee PS, Ghosh A, Ghosh S, Mridha AR et al (2010) Nonobese population in a developing country has a high prevalence of non-alcoholic fatty liver and significant liver disease. Hepatology. 51(5):1593–1602
Wei JL, Leung JC, Loong TC, Wong GL, Yeung DK, Chan RS et al (2015) Prevalence and severity of non-alcoholic fatty liver disease in non-obese patients: a population study using proton-magnetic resonance spectroscopy. Am J Gastroenterol 110(9):1306–1314
Cruz AC, Bugianesi E, George J, Day CP, Liaquat H, Charatcharoenwitthaya P et al (2014) Characteristics and long-term prognosis of lean patients withnon-alcoholic fatty liver disease. Gastroenterology. 146(5):S-909
Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E et al (2011) Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology. 54(3):1082–1090
Ballestri S, Mantovani A, Byrne C, Lonardo A, Targher G. Diagnostic accuracy of ultrasonography for the detection of hepatic steatosis: an updated meta-analysis of observational studies. Metabolism and Target Organ Damage 2021 Sep 7;1(7)
EASL-EASD-EASO (2016) Clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 64(6):1388–1402
Ryan CK, Johnson LA, Germin BI, Marcos A (2002) One hundred consecutive hepatic biopsies in the workup of living donors for right lobe liver transplantation. Liver Transpl 8(12):1114–1122
Haberal M, Telatar H, Demirag A, Boyacioğlu S, Bilgin N (1995) Is needle biopsy mandatory for donor selection in live donor segmental liver transplantation? Transplant Proc 27(5):2605–2606
Bashir S, Mittal K, Khisti R, Yadav A, Mukund A, Pamecha V (2018) Portal vein thrombosis after donor liver biopsy: case report. The Indian journal of radiology & imaging 28(1):61
Hwang S, Lee SG, Jang SJ, Cho SH, Kim KH, Ahn CS et al (2004) The effect of donor weight reduction on hepatic steatosis for living donor liver transplantation. Liver Transpl 10(6):721–725
Nakamuta M, Morizono S, Soejima Y, Yoshizumi T, Aishima S, Takasugi SI et al (2005) Short-term intensive treatment for donors with hepatic steatosis in living-donor liver transplantation. Transplantation. 80(5):608–612
Ozawa Y, Tamura T, Owada Y, Shimizu Y, Kemmochi A, Hisakura K et al (2018) Evaluation of safety for hepatectomy in a novel mouse model with nonalcoholic-steatohepatitis. World J Gastroenterol 24(15):1622
Hoppe S, von Loeffelholz C, Lock JF, Doecke S, Sinn BV, Rieger A et al (2015) Nonalcoholic steatohepatitis and liver steatosis modify partial hepatectomy recovery. J Investig Surg 28(1):24–31
Buechter M, Thimm J, Baba HA, Bertram S, Willuweit K, Gerken G et al (2019) Liver maximum capacity: a novel test to accurately diagnose different stages of liver fibrosis. Digestion. 100(1):45–54
Alizai PH, Lurje I, Kroh A, Schmitz S, Luedde T, Andruszkow J et al (2019 Feb) Noninvasive evaluation of liver function in morbidly obese patients. Gastroenterol Res Pract 3:2019
Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM et al (2006) Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol 24(13):2065–2072
Reddy SK, Marsh JW, Varley PR, Mock BK, Chopra KB, Geller DA et al (2012) Underlying steatohepatitis, but not simple hepatic steatosis, increases morbidity after liver resection: a case-control study. Hepatology. 56(6):2221–2230
Yamamoto K, Takada Y, Fujimoto Y, Haga H, Oike F, Kobayashi N, et al. Nonalcoholicsteatohepatitis in donors for living donor liver transplantation. Transplantation 2007; 83(3):257–62
Pamecha V, Bharathy KG, Mahansaria SS, Sinha PK, Rastogi A, Sasturkar SV (2018) “No go” donor hepatectomy in living-donor liver transplantation. Hepatol Int 12(1):67–74
Taitano AA, Markow M, Finan JE, Wheeler DE, Gonzalvo JP, Murr MM (2015) Bariatric surgery improves histological features of non-alcoholic fatty liver disease and liver fibrosis. J Gastrointest Surg 19(3):429–437
Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR et al (2010) Randomized controlled trial testing the effects of weight loss on non-alcoholic steatohepatitis. Hepatology 51(1):121–129
Golabi P, Locklear CT, Austin P, Afdhal S, Byrns M, Gerber L et al (2016) Effectiveness of exercise in hepatic fat mobilization in non-alcoholic fatty liver disease: systematic review. World J Gastroenterol 22(27):6318–6327
Availability of data and material
The data that support the findings of the present study are available within the article.
Author information
Authors and Affiliations
Contributions
Study concept: Viniyendra Pamecha. Study design: Viniyendra Pamecha, Nilesh Patil, Piyush Kumar Sinha. Data collection: Kumaraswamy Parthasarathy, Nihar Mohapatra, Archana Rastogi, Karthika Rudrakumar, Ashok Choudhury, Amar Mukund, Uma Kanal. Analysis and interpretation of data: Nilesh Patil, Kumaraswamy Parthasarathy, Viniyendra Pamecha, Archana Rastogi.
Manuscript drafting: Nilesh Patil, Viniyendra Pamecha, Kumaraswamy Parthasarathy, Archana Rastogi. Critical revision of the manuscript for important intellectual content: Viniyendra Pamecha, Nilesh Patil.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the Institutional Review Board (no. IEC/2020/81/NA01) of Institute of Liver and Biliary Sciences, New Delhi and performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Conflict of interest
The authors declare no competing interests.
Consent to participate
Informed consent was obtained from all individuals as a mandatory part of quality control in our hospital.
Consent for publication
All authors have read the final version of the article and have provided consent for the article to be published in Langenbeck’s Archives of Surgery.
Research registration unique identifying number (UIN)
1. Name of the registry: ClinicalTrials.gov. Registration: https://www.clinicaltrials.gov/
2. Unique identifying number or registration ID: NCT04571957.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper was presented as an oral presentation at the 25th Annual Scientific Meeting of the Indian National Association for Study of The Liver at New Delhi, India, in August 2017.
Rights and permissions
About this article
Cite this article
Pamecha, V., Patil, N.S., Parthasarathy, K. et al. Expanding donor pool for live donor liver transplantation: utilization of donors with non-alcoholic steatohepatitis after optimization. Langenbecks Arch Surg 407, 1575–1584 (2022). https://doi.org/10.1007/s00423-022-02444-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-022-02444-5